May marks Women’s Health Month, dedicated to raising awareness about women’s health issues, promoting screenings and preventative care, and empowering women to make informed decisions about their overall health and well being. Within the research field, sex differences have historically been overlooked and understudied, limiting our understanding of how biological differences impact physiological processes, disease susceptibility, and treatment responses. However, in recent years, growing recognition has led to increased research efforts exploring sex differences. As such, this month’s publication review highlights the latest female-focused studies in the muscle physiology field.
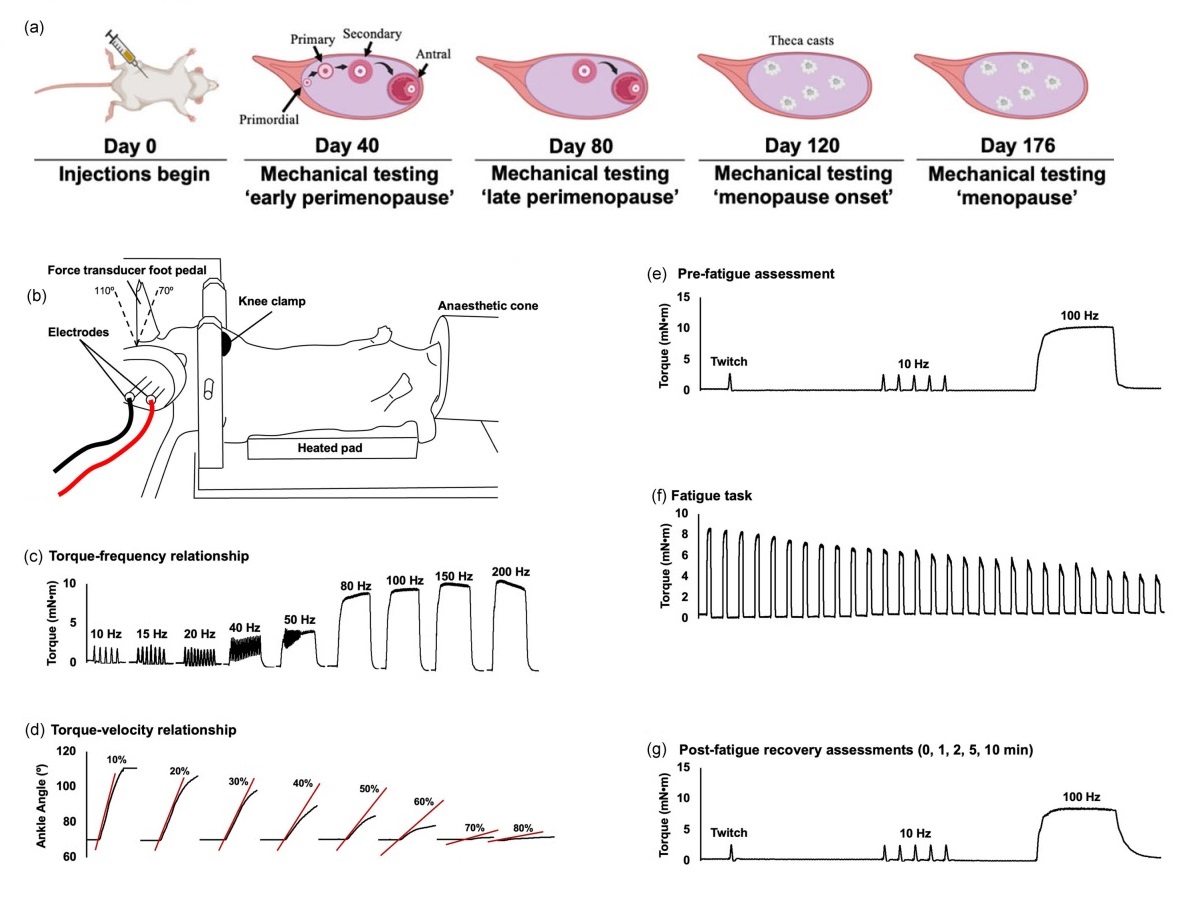
Featured image (adapted from ©Hinks et al. (2024), licensed under CC BY 4.0 DEED) outlining the in-vivo experimental set-up and timeline. A) Injection and testing timeline. B) Diagram of in-vivo set-up. C) Protocol for torque-frequency relationship, D) Torque-velocity relationship, E) Pre-fatigue assessment, F) Fatigue task, and G) Post-fatigue recovery.
Effects of chemically induced ovarian failure on single muscle fiber contractility in a mouse model of menopause
Menopause is defined by the end of menstruation and a decline in ovarian follicular activity, particularly in estradiol production, which can negatively impact muscle function. In the past, researchers used ovariectomized rodent models to explore the effects of menopause on muscle contractility; however, these models did not accurately reflect the natural course of menopause, which is accompanied by a host of hormonal changes. To address this, Mashouri et al. (2023) employed a 4-vinylcyclohexene diepoxide (VCD)-induced gradual ovarian failure mouse model to explore the effect of perimenopausal transition on muscle contractility.
Specifically, the SOL and EDL muscles were harvested and chemically permeabilized in preparation for assessment with Aurora’s Scientific’s 1400A permeabilized fiber system. Single fibers were then tied to pins between the 403A force transducer and 322C high-speed length controller, and sarcomere length was measured using the high-speed 900B VSL program. Across the 8-well bath plate of Aurora Scientific’s 802D permeabilized fiber apparatus, the fibers were submerged in varying pCa levels (ranging from 7.0–4.5) to establish a force-pCa relationship.
Upon analysis, Mashouri et al. (2023) found that alterations to muscle contractility were less evident in ovariectomized models. Moreover, the rate of force redevelopment of single fibers from the SOL was faster in the ovarian failure group, and Type I fibers from the SOL exhibited higher calcium sensitivity compared to controls. Overall, these findings suggest that menopause-induced alterations in muscle contractility may differ from ovariectomized models, underscoring the importance of more accurately modelling the natural progression of menopause, and assessing its impact on neuromuscular function.
Time course changes in in vivo muscle mechanical function and Ca2+ regulation of force following experimentally induced gradual ovarian failure in mice
On a similar note, Hinks et al. (2024) explored how gradual ovarian failure, as opposed to abrupt cessation, affects muscle contractility across different stages of menopause (peri-, early, and late-stage). While it is known that abrupt loss of ovarian hormones negatively impacts muscle function, the effects of the more gradual hormone decline experienced during the natural progression of menopause have not been thoroughly investigated.
Using the same VCD-induced ovarian failure mouse model, the plantarflexors of the female mice were assessed in-vivo using the 300C-LR dual-mode lever system and 809C in situ mouse apparatus of Aurora Scientific’s 1300A whole animal system at 40 (early perimenopause), 80 (late perimenopause), 120 (menopause onset) and 176 (late menopause) days post-initial VCD injection.
Results showed that gradual ovarian failure caused minimal detriments to muscle function, with only minor alterations observed primarily in low-frequency stimulation during recovery from fatigue. VCD mice had 16% lower twitch torque than controls, but other recovery measures showed no significant differences. Taken together, these longitudinal in-vivo assessments provide a more physiologically relevant assessment of the changes in muscle mechanical function during the development of menopause.
Characterizing the effects of muscle-specific GSK3a/b reduction on murine muscle contractility and metabolism in female mice
Glycogen synthase kinase 3 (GSK3) is an enzyme involved in skeletal muscle physiology and metabolism. It has importantly been implicated in the control of several signaling pathways, many of which affect muscle size. Although numerous studies examining the role of GSK3 in muscle physiology have made valuable contributions to our understanding, most have been exclusively conducted on male mice, leaving the effects of GSK3 inhibition in female mice largely unknown. Here, Hockey et al. (2024) investigated the effects of partial knockdown of GSK3 in female mice to better understand how sex difference may impact functional outcomes.
Using Aurora Scientific’s 1200A isolated muscle system, both the EDL and SOL muscles of female mice were isolated for contractility and fatigue assessments.
While previous research in male mice showed increased lean mass and activation of muscle-based adaptive thermogenesis, the results in female mice differed significantly. Muscle-specific GSK3 knockdown did not alter body composition, energy expenditure, glucose tolerance, or mitochondrial respiration in female mice. Additionally, the observed improvements in muscle strength and fatigue resistance previously seen in male mice were not replicated in the females, highlighting critical sex differences in the response to genetic reduction of GSK3.
Conclusions
These studies by Mashouri et al. (2023), Hinks et al. (2024), and Hockey et al. (2024) collectively advance our understanding of how sex differences can impact muscle physiology, and prime the field for further investigation into disease progression, treatment, and intervention strategies for women. Although historically overlooked and understudied, these strides within the field May just mark the start of more female-focused research.