In the context of muscle, the field of biomechanics explores how muscles generate force, produce movement, and interact with the surrounding muscle architecture, such as bones and tendons. Biomechanical researchers often analyze the mechanical properties of muscle tissues, such as tension, elasticity, and stiffness, and delineate fiber orientation and contraction dynamics. These assessments are critical to understanding how muscles function during movement and how injuries arise, as well as informing rehabilitative practices and therapeutic strategies. The following publication review features recent advances in this field – ranging from the mechanical properties of rotator cuff muscles to the impact of lengthening and force velocities on different muscle groups.
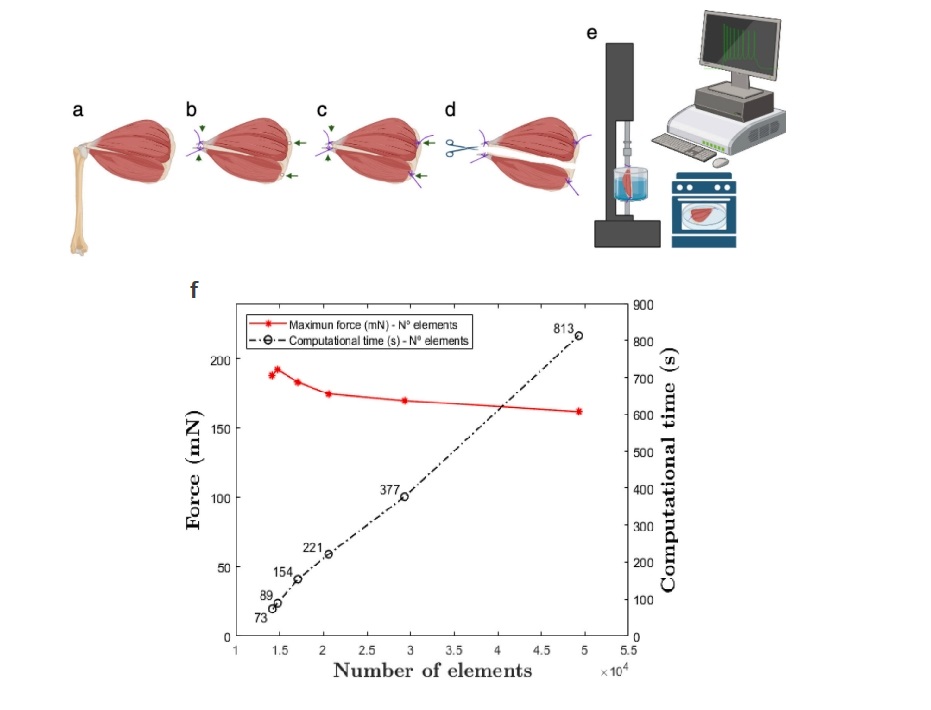
Featured image (adapted from ©Heras-Sádaba et al. (2024), licensed under CC BY-NC 4.0 DEED) depicting a scheme of the samples preparations. A) Supraspinatus and infraspinatus tendons inserted on the humeral head, B) free tendons secured with sutures (arrowheads), C) fixation with suture loops (arrows), D) isolation of the individual muscles, E) experimental setup, and F) a graph with the relationship between the maximum force developed by the infraspinatus muscle in the model (mN), the number of elements in the mesh, and the computational time (s) for each simulation.
Exploring the muscle architecture effect on the mechanical behaviour of mouse rotator cuff muscles
The rotator cuff (RC) is composed of the supraspinatus, infraspinatus, subscapularis, and teres minor muscles. Although the RC is known for its crucial role in maintaining the motion and stability of the shoulder joint, it is often susceptible to injury, leading to pain, weakness, a reduced range of motion, and functional limitations. Due to this, extensive research has been conducted on the supraspinatus muscle anatomy, but relatively few studies go on to explore the infraspinatus–supraspinatus relationship. Given how muscular architecture intricately underlies the functional behaviour of a muscle group, the mechanical properties and architectural differences between the supraspinatus and infraspinatus muscles can help delineate muscle behavior during movement and injury. Therefore, Heras-Sádaba et al. (2024) sought to investigate the mechanical properties of the supraspinatus and infraspinatus muscles in mice.
To achieve this, they isolated the supraspinatus and infraspinatus muscles from twelve wildtype C57BL/6J mice for histological and mechanical characterization. The tissue sections were stained with Hematoxylin and Eosin, Mason’s Trichromic and Sirius red staining to uncover the histological structure and quantify the collagen content. Aurora Scientific’s 1200A isolated muscle system was used to perform contractile force generation tests, in which the supraspinatus and infraspinatus muscles were mounted, set at their optimal length, and stimulated with both isometric twitch pulses and tetanic contractions. A finite element model was then used to characterize the mechanical behaviour of the muscles by integrating histological data and fiber orientation.
Analysis of the histological stains revealed that the infraspinatus muscle had significantly higher collagen content than the supraspinatus, which exhibited a stiffer passive behavior. Additionally, the analysis of muscle fiber orientations revealed that the pennation angle plays a crucial role in the mechanical behavior of these muscles, with higher angles correlating to greater force production. The integration of multiphoton microscopy and finite element modeling allowed for detailed visualization and modeling of muscle architecture. Together, these findings provide valuable insights into shoulder biomechanics and can critically inform clinical approaches to injuries and rehabilitation.
Impact of lengthening velocity on the generation of eccentric force by slow‑twitch muscle fibers in long stretches
Within literature, the phenomenon known as Give, refers to a temporary reduction in muscle force during an eccentric contraction after an initial peak force. This occurs during the early phase of muscle stretching, where the force generated by the muscle drops before subsequently increasing again. While previous research has focused on fast-twitch fibers and established the concept of Give, the exact mechanisms and velocity-dependent characteristics of this phenomenon in slow-twitch fibers has yet to be explored. To address this, Weidner et al. (2024) investigated how stretch velocity affects contractility in slow-twitch (soleus) muscle fibers.
Soleus muscles were isolated from the left hind limb of male Wistar rats, permeabilized in skinning solution and clamped on both sides with aluminum foil T-shaped clips. The fibers were then attached to Aurora Scientific’s 1400A permeabilized fiber system, including the 403A force transducer and 322C high-speed length controller, for the measurement of force and length changes during eccentric stretching at various velocities. Data acquisition was performed using the 600A real-time muscle data acquisition and analysis system, enabling the tracking of sarcomere lengths and force responses, which were subsequently analyzed using MATLAB.
Interestingly, the slow-twitch soleus fibers generated a maximum isometric tension of 98.6 kN m−2 at optimal length and a maximum contraction speed of 0.47 lopt s−1, with significant force increases during isovelocity stretches at higher velocities. The force response included an initial steep increase, Give, and a subsequent recovery, with all parameters significantly affected by stretch velocity. Notably, soleus fibers exhibited unique behavior, including a consistent occurrence of Give across all tested velocities, in contrast to fast-twitch fibers (EDL), which only showed Give at higher velocities. Overall, the findings highlight both similarities and distinct differences in force generation dynamics between slow- and fast-twitch muscle fibers during stretch.
The impacts of muscle-specific force-velocity properties on predictions of mouse muscle function during locomotion
Musculoskeletal models aid researchers and clinicians alike by providing valuable insights into muscle function, injury mechanics, and the prediction of how different components of muscular architecture work together during movement. While valuable, the use of generalized force-velocity relationships in existing musculoskeletal models can limit the accurate prediction of muscle function during locomotion. To assess the accuracy of these models, Charles et al. (2024) investigated the effects of muscle-specific force-velocity properties on predictions of mouse muscle function during locomotion.
To achieve this, a previously validated musculoskeletal model of the mouse hindlimb and pelvis was employed, followed by ex-vivo validation on isolated muscles. Both the soleus (SOL) and extensor digitorum longus (EDL) muscles were dissected from the hindlimb of male C57BL/6 mice, and aluminum clips were attached to the proximal and distal tendons. These clips allowed for attachment to Aurora Scientific’s 300B-LR dual mode motor, while the muscles were submerged in the vertical bath of the 800A in-vitro muscle apparatus. The 701C electrical stimulator and 610A Dynamic Muscle Control Software were used together to stimulate the muscles according to the custom-written protocols.
Upon analysis, the incorporation of muscle-specific force-velocity properties were found to significantly alter the predicted functional outputs of mouse muscles during locomotion. The EDL produced more positive work and exhibited spring-like behavior, while the SOL generated more negative work and functioned as a brake. Additionally, applying these specific properties to other muscles classified as fast or slow substantially influenced their predicted functions, enhancing alignment with experimental data. These results suggest that tailored force-velocity relationships indeed improve the accuracy of musculoskeletal models in simulating muscle dynamics.
Conclusions
These studies by Heras-Sádaba et al. (2024), Weidner et al. (2024), and Charles et al. (2024) showcase how specific anatomical and physiological properties—such as rotator cuff mechanics, lengthening velocities, and force-velocity relationships—critically impact locomotion and muscle performance. Collectively, their findings can be applied to improve the accuracy of musculoskeletal models and inform future therapeutic avenues in the field.