In both cardiac and skeletal muscle, myofilaments are key regulators of muscle contraction with thick-thin filament interactions producing force and motion. Many myofilament mechanisms underlying disease pathogenesis, however, are poorly understood. Aurora Scientific offers many technologies and instruments for facilitating mechanistic studies, some of which are highlighted below in this publication review.
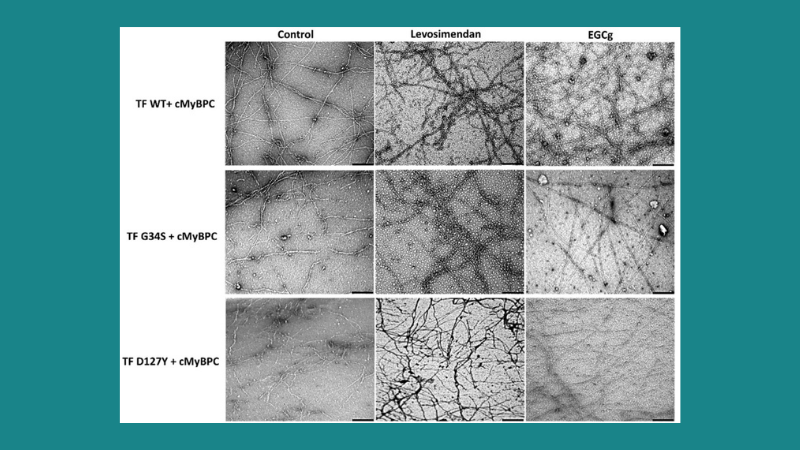
Featured image demonstrates the therapeutic effects of the troponin targeting agents levosimendan and green tea extract (-)-epigallocatechin-3-gallate (EGCg) on the structure and morphology of thin filaments in troponin mutations. (© 2021 Hassoun, R. et al.). Scale bars represent 250 nm.
De Novo Missense Mutations in TNNC1 and TNNI3 Causing Severe Infantile Cardiomyopathy Affect Myofilament Structure and Function and are Modulated by Troponin Targeting Agents
Pediatric cardiomyopathies (CMs) are rare cardiac muscle disorders that are associated with poor prognoses, and the mechanisms underlying some uncommon pediatric CMs such as non-compaction (NCM) and restrictive (RCM) are poorly understood. To address this research gap, Hassoun et al. studied two de novo point mutations in TNNC1 and TNNI3 in infants with end stage heart failure of NCM and RCM, respectively. Using skinned cardiomyocytes, skinned fibers, and reconstituted thin filaments, the authors measured the effects of the mutations on contractile function. Force recordings of skinned cardiomyocytes from patient and human donor hearts were performed with our 1600A permeabilized myocyte system and 403A force transducer. While each patient developed a different phenotype, both mutations demonstrated similar functional and structural impairments. Since specific pharmacological treatments have yet to be identified, the authors also investigated two troponin targeting agents, levosimendan and EGCg, to determine if contractile function and thin filament could be restored; both drugs modulated contractile function in vitro as well as the structural integrity of reconstituted thin filaments.
Sarcomeric Deficits Underlie MYBPC1-Associated Myopathy with Myogenic Tremor
MYBPC1, expressed in skeletal muscles, encodes myosin binding protein-C slow and contributes to myosin thick filament stabilization and actomyosin cross-bridge regulation. The causal association of dominant missense pathogenic variants in MYBPC1 with early-onset myopathy characterized by muscle weakness, hypotonia, skeletal deformities, and tremor was recently identified. To determine the mechanisms by which the E248K pathogenic variant drives disease pathogenesis, Hauserman et al. generated an E248K knock-in (KI) mouse model using CRISPR/Cas9 technology. Ex vivo contractility studies were performed with our 300B-LR lever system operated in isometric mode, while skinned single-fiber mechanical measurements were performed using our 403A force transducer and 308C length controller. The heterozygous E248K KI variant was shown to recapitulate the myopathic phenotype, tremor, and disease onset and progression that is observed in human E248K carriers. The authors also demonstrated that the loss-of-function phenotype is mainly driven by disordered and misaligned sarcomeres by performing biochemical, ultrastructural, and contractile assessments of the tissue, cell, and myofilaments. The authors concluded that the findings from this study provide mechanisms for this disease pathogenesis and offer a relevant preclinical model for facilitating therapeutic discovery.
A Mechanism for Sarcomere Breathing: Volume Change and Advective Flow Within the Myofilament Lattice
Myosin motors bind to and slide actin thin filaments during muscle contraction and rely on energy derived from ATP. The radial spacing of these lattice filaments may change or remain the same during contraction. If isovolumetric, the lattice must expand when the muscle shortens, but if the spacing is constant or has a different pattern of axial and radial motion, the lattice will change volume during contraction. Cass et al. created an advective-diffusive-reaction flow model and showed that the flow through the sarcomere lattice would be significant without lattice expansion. Force measurements for this study were performed with our 305C dual-mode lever. The authors used time-resolved x-ray diffraction of contracting muscle to demonstrate that the contractile lattice is neither isovolumetric nor constant in spacing, but time-varying and activation-dependent. Fluid flow in the sarcomere lattice of synchronous insect flight muscles was greater than expected for constant lattice spacing conditions, and modelling demonstrated that these flows could deliver a significant advective advantage over the passive diffusion of ATP. Although muscle type and contraction dynamics influence its magnitude, advective flow due to muscle contraction is a previously unrecognized mechanism that can influence energy delivery, substrate exchange, and regulatory molecule flux in the myofilament lattice.