As the air turns crisp and October casts its spell, it’s the perfect time to uncover spooky-good science from the world of skeletal muscle. This season, we’re diving into the realm of fast-tWitches, bone-chilling skeletal muscle mysteries, and ghost fibers. From fucoidan extracts that ward off heat-induced muscle decline, to neural signals that haunt the mTORC1 pathway, and eerie insights into mantATP turnover in “ghosted” fibers, researchers are conjuring up a cauldron of compelling discoveries in this Halloween-themed publication review.
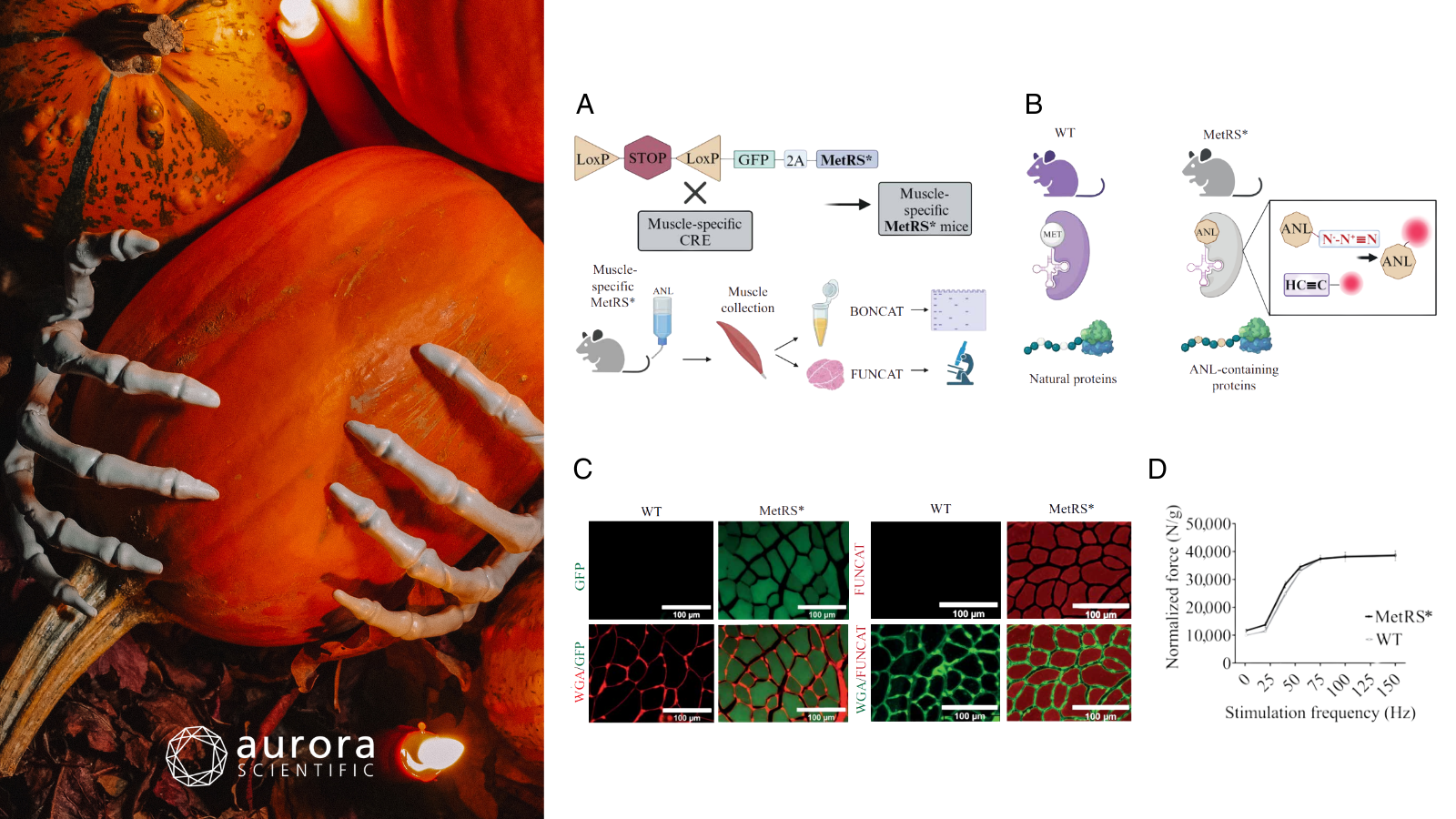
Featured image includes a pumpkin with skeleton hands (©bilder-von-yuliya-meshkova via Canva.com) and adapted figures from ©Dumitras et al (2025), licensed under CC BY 4.0. A) Overview of the generation of muscle-specific MetRS mice and the workflow for labeling, identifying, and visualizing newly synthesized proteins; B) Diagram illustrating the difference between the mutated MetRS enzyme and wildtype (WT) MetRS; C) Representative diaphragm muscle sections with fibers colour-coded by cross-sectional area (CSA); scale bar = 100 µm; D) Muscle cryosections stained for GFP (left panels: GFP in green, WGA in red) and FUNCAT (right panels: FUNCAT in red, WGA in green) after 7 days of labeling in MetRS mice versus WT controls; E) Comparison of force production in MetRS* and WT mice following electrical stimulation of the sciatic nerve.
The impact of fucoidan extracts on heat-stress-induced loss of in vitro fast-twitch muscle function in mice
High temperatures have long intrigued muscle physiologists due to their detrimental effects on muscle force production, particularly in fast-twitch fibers. While oxidative stress has been implicated in this decline, only a handful of studies have explored antioxidant-based strategies to counteract heat-induced dysfunction. Fucoidan, a sulfated polysaccharide found in brown seaweed, has emerged as a compound of interest due to its potent antioxidant properties, yet its effects on skeletal muscle under heat stress remain unknown. In this study, Kucewicz et al (2025) set out to investigate whether fucoidan derived from Fucus vesiculosus or Undaria pinnatifida could protect against temperature-induced muscle weakness.
Male C57BL/6 mice were orally administered fucoidan or vehicle control for seven days before isolated fast-twitch extensor digitorum longus (EDL) muscles were harvested for in-vitro testing. Muscles were mounted in a temperature-controlled organ bath and stimulated to contract at either 25°C or 43°C to assess force production under heat stress. Muscle performance was measured using Aurora Scientific’s 1300A Whole Animal System for Mice, including the 300C-LR Dual-Mode Muscle Lever to capture contractile force and 615A: Dynamic Muscle Control and Analysis Software to deliver stimuli and record responses. Additional analyses included gene expression via qRT-PCR and reactive oxygen species (ROS) quantification in C2C12 myoblasts subjected to heat stress.
Exposure to 43 °C resulted in a significant and progressive decline in EDL muscle force production compared to muscles maintained at 25 °C. However, mice treated with fucoidan from Fucus vesiculosus (FVF) showed a marked attenuation of this force loss during the early stages of heat stress, while Undaria pinnatifida fucoidan (UPF) offered no such benefit. Heat exposure also elevated the expression of several heat shock proteins (HSP27, HSP70, and HSP90), though only FVF treatment further increased HSP90 expression. Additionally, both fucoidan types prevented a heat-induced rise in ROS in C2C12 cells, suggesting a shared antioxidant capacity despite their differing effects on muscle force preservation. These findings underscore the potential of marine-derived fucoidans as targeted nutritional interventions to enhance muscle resilience under thermal stress.
Neural stimulation suppresses mTORC1-mediated protein synthesis in skeletal muscle
Muscle size and function are shaped by fiber type and activity level, with oxidative (slow-twitch) fibers generally smaller and more resistant to wasting than glycolytic (fast-twitch) ones. While the mTORC1 pathway is known to regulate muscle growth, how it operates differently across fiber types in response to nerve activity has remained a mystery, largely due to the lack of fiber-specific tools to track protein synthesis in-vivo. Dumitras et al (2025) therefore introduced a transgenic mouse model that enables fiber-type-specific labeling of newly synthesized proteins via a modified tRNA synthetase and click chemistry.
Inducible muscle-specific MetRS* mice were generated and treated with tamoxifen to enable in-vivo labeling of newly synthesized proteins using azidonorleucine (ANL). Mice underwent denervation, rapamycin treatment, and electrical stimulation, followed by analyses including BONCAT/FUNCAT labeling, Western blotting, histology, and proteomics. Muscle contractile performance was then assessed in-vivo using Aurora Scientific’s 305B Dual-Mode Muscle Lever System, which measured gastrocnemius force to evaluate functional outcomes alongside molecular analyses.
Muscle-specific MetRS* mice showed efficient labeling of newly synthesized proteins without affecting contractile performance, as force measurements confirmed normal muscle strength. Denervation led to a 15–20% decrease in muscle mass but an mTORC1-dependent increase in protein synthesis in mitochondria-rich type 1 and 2A fibers, while type 2B fibers showed reduced labeling. Conversely, electrical stimulation suppressed protein synthesis and mTORC1 signaling, indicating that muscle activity transiently inhibits anabolic processes. These results demonstrate that mTORC1 activity and protein synthesis in muscle fibers are finely tuned by nerve activity and fiber type.
A combined experimental and computational analysis of mantATP turnover in skinned muscle fibers
Myosin, the motor protein driving muscle contraction, also consumes ATP at rest through two states: the Super Relaxed State (SRX) and the Disordered Relaxed State (DRX). These states are commonly studied using mantATP chasing, which tracks fluorescent nucleotide turnover. However, recent studies have questioned the accuracy of this method in soluble myosin preparations due to artifacts from nonspecific binding and diffusion limitations. While skinned muscle fibers preserve native myosin organization and cooperativity, these same features complicate mantATP data interpretation. To address this, Montesel et al (2025) combined experimental measurements with computational modeling to separate specific and nonspecific components of the mantATP signal and improve SRX/DRX quantification in skinned fibers.
A Monte Carlo sarcomere model simulated myosin cooperativity and SRX/DRX transitions, while a reaction–diffusion model described mantATP hydrolysis and diffusion within fibers. Experimentally, single permeabilized fibers were mounted in an Aurora Scientific 1400A Permeabilized Fiber System, equipped with the 802D Permeabilized Fiber Apparatus, a 403C Force Transducer, and 315D High-Speed Length Controller, allowing precise sarcomere-length control and mechanical monitoring during mantATP chasing. Confocal microscopy visualized mantATP binding, mass spectrometry assessed protein composition, and NADH-coupled ATPase assays measured ATP hydrolysis rates.
Results demonstrated that myosin cooperativity is essential to reproduce the biphasic mantATP fluorescence decay corresponding to SRX and DRX states. Reaction–diffusion simulations confirmed that diffusion and nonspecific binding contribute to slower decay phases, supporting a three-exponential fitting model for accurate analysis. MantATP chasing and ATPase assays in intact fibers captured nucleotide turnover changes, including those induced by piperine. In ghosted fibers (where myosin has been largely removed) approximately 60% of the mantATP signal arose from nonspecific interactions, providing a reference for correcting fluorescence data in myosin-depleted preparations. This integrated experimental–computational framework clarifies how myosin cooperativity and nonspecific nucleotide interactions shape mantATP signals, enabling more accurate measurements of SRX and DRX states and assessment of muscle energetics modulators.
Conclusions
These studies by Kucewicz et al (2025), Dumitras et al (2025), and Montesel et al (2025) reveal how skeletal muscle is shaped by both subtle and “ghostly” forces. From fucoidan shielding fast-twitch fibers, to neural signals haunting mTORC1 activity, to nonspecific mantATP signals in ghosted fibers, this work reminds us of the unseen forces that quietly govern muscle energetics.