Rodents may be the most common laboratory animal worldwide, but did you know that even insects can advance muscle physiology and neurological research? At Aurora Scientific, we provide instrumentation to help assess muscle biomechanics in a variety of animal models from flies to octopuses, and highlight some recent examples in this publication review.
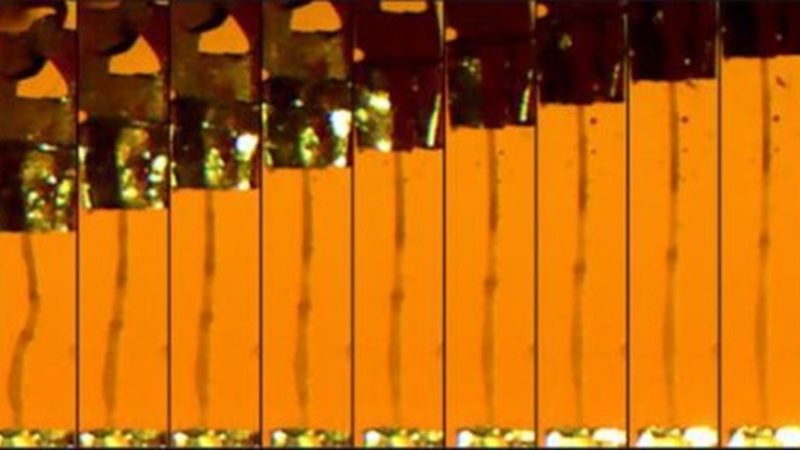
Featured image (© 2021 Niosi et al., licensed under CC BY 4.0) represents microscopy image time lapse of a dissected midgut from a fruit fly undergoing mechanical testing.
Distributed Processing of Load and Movement Feedback in the Premotor Network Controlling an Insect Leg Joint
Proprioception is critical for motor control in legged animals. In insects, proprioceptive feedback is provided by chordotonal organs, which monitor the position and movement of the femur-tibia joint, and campaniform sensilla, which are used to measure forces and loads that act in different directions on the leg. While neuronal pathway topology is well understood, little is known about how multimodal proprioceptive feedback is integrated in the local networks that control limb movements. Therefore, Gebehart et al. used the common stick insect to investigate the premotor network architecture that underlies multimodal proprioceptive integration, as their size allows for the precise mechanical stimulation of multiple organs, either individually or concurrently.
In this study, the authors found that load feedback from tibial campaniform sensilla affects movement-elicited resistance reflex strength and determines the specificity of how extensor tibiae and retractor coxae respond to load and movement stimuli controlled with Aurora’s 300C Dual-Mode lever system. Additionally, the authors observed multimodal sensory signal interaction at every level of single nonspiking interneurons and motor neurons. Taken together, these results suggest that load and movement feedback are integrated in a multimodal local premotor network composed of elements that control femur-tibia joint movements. The authors conclude that this study significantly expands current knowledge on how legged motor systems achieve finely-tuned motor control.
Kismet/CHD7/CHD8 Affects Gut Biomechanics, the Gut Microbiome, and Gut-Brain Axis in Drosophila melanogaster
The gut-brain axis is a communication loop between the gut microbiome and the brain that is crucial for both digestion and neurological function, and so determining how neurodevelopmental-associated genes affect gut physiology could expand treatment options for both gastrointestinal and behavioral symptoms. Due to the simplicity of their tissues and gut microbiome, as well as their intestinal pathophysiological similarities to mammals, the common fruit fly is being increasingly used to model the gut-brain axis. Fruit flies also possess genes that are orthogonal to risk genes for human neurodevelopmental disorders that have co-occurring gastrointestinal symptoms, such as kismet, the ortholog to mammalian chromodomain helicase DNA-binding domain protein (CHD) genes, CHD7 and CHD8. Thus, using fruit flies, Niosi et al. have investigated how these risk genes affect gut physiology, and employed our 322C-I High-Speed Length Controller and 403B Force Transducer for gut tissue mechanical measurements.
The authors found significant changes in the mechanics of kismet mutant guts in terms of elasticity and tensile strength, as well as reduced gut microbiota diversity and increased pathogenic taxa. Interestingly, depleting gut microbiota improved courtship defects in kismet mutant flies, indicating that there is a connection between the gut microbiome and behavior. In contrast, gut microbiota depletion in control flies reduced courtship activity, demonstrating that antibiotic treatments can differentially affect behavior based on gut microbial dysbiosis status. The authors conclude that kismet-mediated changes in gut structure, mechanics, and function have important roles in the gut-brain axis, and suggest that gut tissue biomechanics should be considered as an element in this communication loop.
How Octopus Arm Muscle Contractile Properties and Anatomical Organization Contribute to Arm Functional Specialization
Octopus arms are muscular hydrostats that can actively elongate, bend, and twist by activating obliquely striated muscles. However, a limited number of studies have functionally investigated central motor control in cephalopods, and so the biomechanics of octopus muscles are not fully understood. Therefore, Zullo et al. investigated the extent to which octopus arm motion can emerge at the limb level by studying the interplay between muscle physiology and anatomical positioning. For this study, biomechanical measurements were performed using our 300C-LR Dual-Mode Lever, while recordings were digitized and analyzed using our 604A Data Acquisition Signal Interface and 605A Dynamic Muscle Data Acquisition System.
The authors demonstrated that longitudinal muscles in octopus arms have a higher activation and relaxation rate, lower twitch-to-tetanus ratio, and lower passive tension compared to transverse muscles, and are thus used as faster and slower muscles, respectively. Additionally, the authors showed the octopus arms have some degree of morphological specialization in which the muscles manifest different biomechanical properties. Findings from this study support that idea that cephalopods are distinguishable animals that have simplified their movement to control hyper-redundant limbs, and the authors conclude that the octopus can be considered as an ensemble of well-coordinated effectors.