There is a new version of this product available. Check out the 300E Dual-Mode Lever Systems!
300C: Dual-Mode Muscle Levers
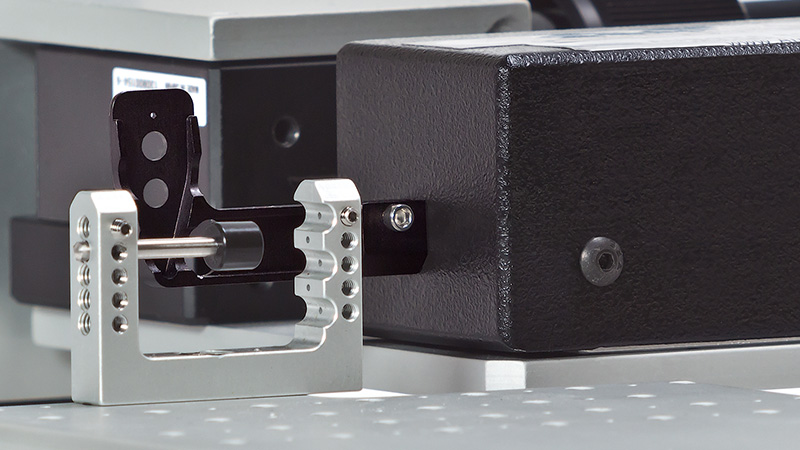
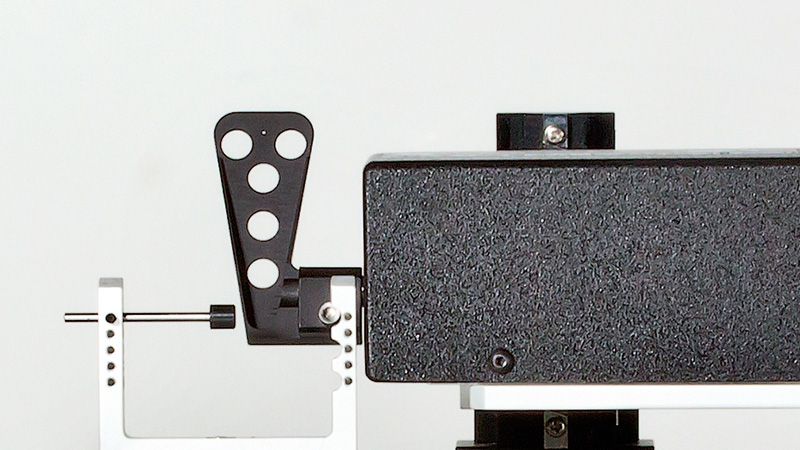
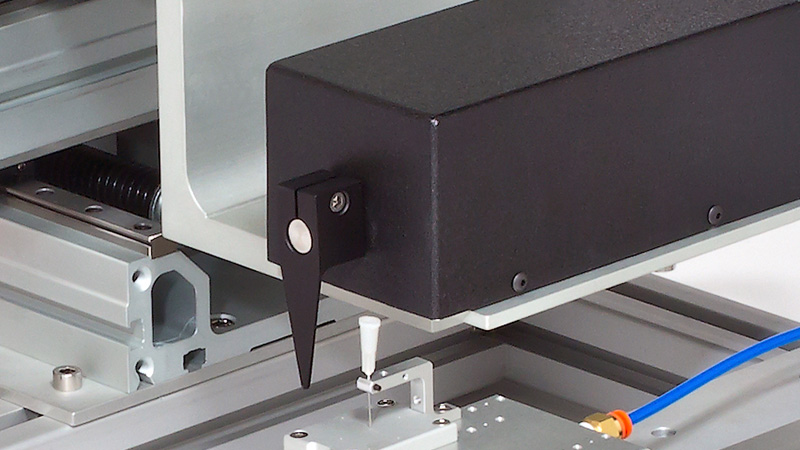
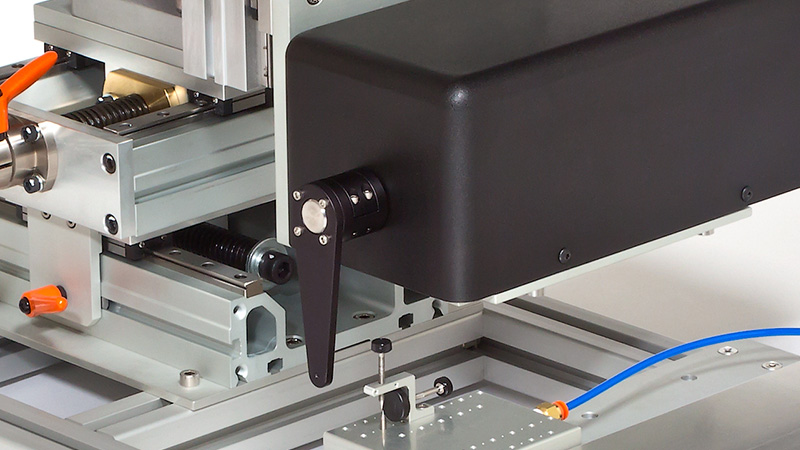
The 300C series of muscle levers are designed with unique specifications that allow the mechanical properties of a number of muscle types and sizes to be characterized. Models are available for small force measurements up to 0.5N (e.g. mouse diaphragm strip or soleus) with a stunning resolution of 0.3mN (300C), up to a maximum force measurement of 100N (e.g. dog hind limb) with a remarkable resolution of 10mN (310C-LR). The length signal resolution of all 7 lever systems is 2.5µm with a step change in length as fast as 1.4ms.
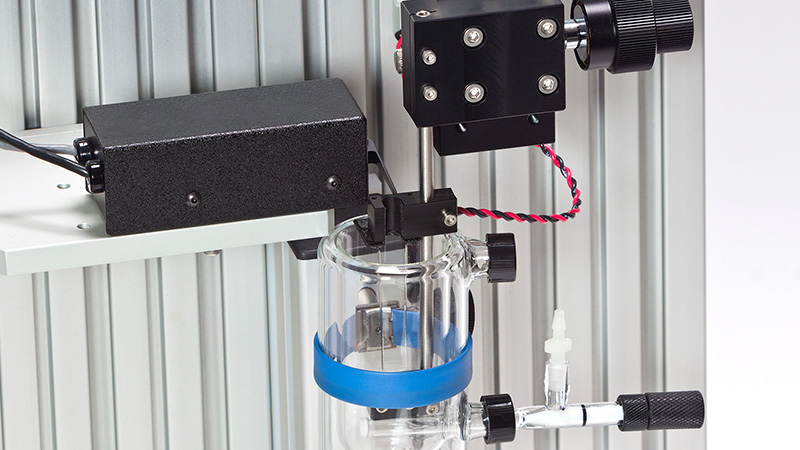
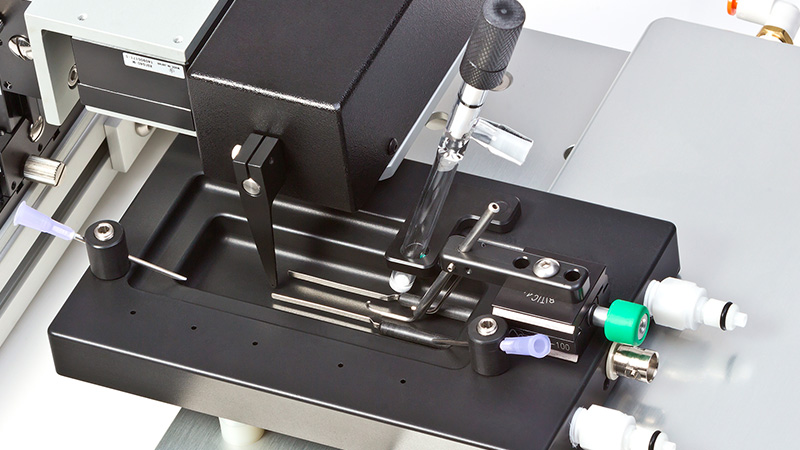
Designed for multiple configurations, researchers can utilize these muscle lever systems for in-vivo, in-situ, and in-vitro preparations. Additionally, the 300C series Dual-Mode levers provide the ability to control both force and length allowing measurement of a number of dynamic muscle properties.
The need for separate force and length systems is eliminated, providing a cost effective, accurate and easy to use solution for the muscle researcher.
Models
300C: 0.5N Force, 10mm Excursion
300C-LR: 1N Force, 10mm Excursion
305C: 5N Force, 20mm Excursion
305C-LR: 10N Force, 20mm Excursion
309C: 20N Force, 30mm Excursion
310C: 50N Force, 40mm Excursion
310C-LR: 100N Force, 40mm Excursion
Variants
Footplate Variants:
Control and measure torque and angle for in-vivo mechanics of plantar and dorsi flexors. All models listed are available in a footplate variant.
Indenter Variants:
300C – I: Mechanical Stimulator
305C – I: Mechanical Stimulator
2 Channel Dual-Mode Muscle Lever Variants:
300D-300-300: 2 Channel Dual-Mode Muscle Levers – both 300C
300D-300-305: 2 Channel Dual-Mode Muscle Levers – one 300C, one 305C
300D-305-305: 2 Channel Dual-Mode Muscle Levers – both 305C
Stories of Success
Bi-Directional Stretch to Assess Colorectal Afferent Activity
CHALLENGE
At the University of Connecticut, a group of researchers have been studying how the peripheral nervous system encodes and processes sensory information with particular interest in sensory afferent relays of pain. Because the group assessed stretch-activated afferent in smooth muscle, particularly the colon, they needed a way to simultaneously stretch the tissue while doing electrophysiological recordings of afferent activity in models of chronic pain.
SOLUTION
Prior experience with our 300C Dual-Mode Lever system had led this group to produce several papers looking at stretch-activated afferent activity, but they now needed a way to bi-directionally stretch smooth muscle tissue to get a more complete picture of how colorectal afferent activity is modulated in chronic pain models. Aurora Scientific consulted with the UConn group about their needs and determined that our 300D 2-channel Dual-Mode muscle lever would be an ideal solution. The 300D has the unique ability to control and measure length and force in a reliable and precise fashion in two different directions using two motors in unison. This was crucial as the researchers needed to lengthen the muscle repeatedly in opposite directions while recording afferent activity.
RESULTS
The Dual-Mode lever allowed this group of researchers to perform reliable and reproducible bi-directional stretching of colorectal tissue while recording sensory afferent firing properties in models of chronic pain. Using an intact nerve-muscle preparation of a dissected colorectum and pelvic nerve, they can study how various mechanical perturbations such as probing, stroking or in the case of our 300D Dual-Mode, stretching, can affect sensory afferent activity. Because of the capabilities provided to them with the 300D, the lab has been able to expand their capabilities to better model true, real life afferent activity in response to smooth muscle stretching. Integrating this information with other techniques in the lab such as optogenetics, molecular biology and MEMS, the group can gain insight into neuromodulators that could be employed to reduce chronic pain by targeting the peripheral nervous system.
Genetic Therapy Research for Rare Myopathies
CHALLENGE
A group of researchers in North Carolina had several colonies of specially bred dogs as a model for muscular dystrophy and other rare myopathic disorders. To test experimental therapies and learn about the disease progression, the researchers required an instrument to test whole animal muscle function. The researchers had used the Aurora 300C series for other applications previously and approached us about a special in-vivo modification for our largest model in the series, the 310C-LR. Although similar modifications had been made for other models in the series, the sheer size of the animals and the modifications required posed a number of engineering challenges. The first prototypes caused vibrations to appear on the signal and rendered the instrument effectively useless.
SOLUTION
After a number of conventional prototypes made from aluminum failed to perform consistently, and with deadlines looming, the R&D team at Aurora Scientific needed to design an unconventional prototype quickly. To lighten the mass of the modified lever, and to make it stiffer and stronger, a carbon fiber material was used on a conventional aluminum holder. Materials were sourced from a local aerospace company with a long history of using carbon fiber material on rockets. The first prototypes were machined and shipped to the laboratory in time to perform mechanics experiments. Aurora Scientific engineers were on site in the lab to assist with initial testing and measurements.
RESULTS
The carbon fiber prototype proved to work successfully and the vibration was eliminated. This allowed the researchers to perform all the measurements required for testing the muscle function of their specially bred animals. These researchers and many of their collaborators have successfully utilized our custom equipment for nearly eight years, facilitating the characterization of disease progression, advancement of life saving research and enabling promising therapies to reach FDA approval. The design has since been refined and become a standard Aurora Scientific product, which has provided researchers studying other large animals such as pigs, sheep and monkeys the ability to characterize muscle mechanics in-vivo.
Myonecrosis Research in Injured Muscle
CHALLENGE
Dr. Cliff Bayer has been studying myonecrosis induced by Group A streptococci (GAS) bacteria at V.A. Medical Center in Boise Idaho. Interestingly, this necrotizing infection develops in sites of muscle injury and is thought to be exacerbated by anti-inflammatory drugs (NSAIDs). Dr. Bayer was interested in assessing the role of NSAIDs in this process, but had no reliable or reproducible way of inducing hindlimb eccentric injury in mice to test this mechanism. Furthermore, this process needed to be performed in-vivo as injection of GAS needed to be done 48h post-injury.
SOLUTION
Dr. Bayer consulted with Aurora Scientific about his needs. We determined that our 300C Dual-Mode Muscle Lever would be an ideal solution. The 300C has the unique ability to control and measure length and force in a reliable and precise fashion. This was crucial as Dr. Bayer needed to lengthen the muscle repeatedly during stimulation while simultaneous measuring force to induce sufficient muscle injury prior to injecting GAS. In addition, Aurora Scientific’s footplate design permitted in-vivo hindlimb testing that was crucial to his studies.
RESULTS
The dual-mode lever allowed Dr. Bayer to perform reliable and reproducible eccentric injury in murine hindlimb muscle. Furthermore, the footplate attachment allowed him to perform in-vivo experiments increasing throughput and permitting injection of GAS 48h post-injury. This led to numerous publications describing the mechanisms involved in necrotizing infections following muscle injury and more effective treatments to replace use of NSAIDs. Dr. Bayer has successfully utilized this motor for nearly a decade and has recently integrated Aurora Scientific’s 1300A 3-in-1 muscle test system along with peripheral equipment and software to further his research.
Studying the Diverse Array of Muscles
CHALLENGE
A group of researchers at Wright State University had been studying Huntington’s Disease and its effects on skeletal muscle physiology in various animal and muscle models. To better understand the mechanisms and progression of symptoms of this disease this group required a device which could help to create experiments that modelled muscle function and activity beyond simple isometric contractions. Due to the diversity of the muscles and species to be studied, however, several different dual mode levers were simply unaffordable at the time.
SOLUTION
After some discussions back and forth to understand experimental design, we determined that the 300D 2 Channel Dual-Mode Muscle Lever would be an ideal solution. The 300D combines any two of our dual-mode muscle levers up to a maximum force range of 10N. Each of these channels operates independently and, like all of our dual-mode lever systems, has the unique ability to control and measure length and force in a reliable and precise fashion. This factor was very important to this group as Huntington’s disease does not necessarily affect the amount of force a muscle can generate, but rather the kinetics of relaxation or muscle shortening.
RESULTS
The 300D 2 Channel Dual-Mode Muscle Lever was easily able to be integrated into the customized pre-existing experimental setup and customized software. The lab has been able to expand their capabilities to better model true, real life muscle activity while performing in depth research on the mechanisms of Huntington’s disease in various muscle models from mice and rats.
Select References
- Charles et al. “The impacts of muscle-specific force-velocity properties on predictions of mouse muscle function during locomotion” Frontiers in Bioengineering and Biotechnology (2024) DOI: 10.3389/fbioe.2024.1436004
- Segales et al. “Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals” Nature communications (2020) DOI: 10.1038/s41467-019-13832-9
- Gorini et al. “Reversible temporally-specific inhibition of muscle using a light-activated chloride channel” bioRxiv (2018) DOI: 10.1101/398156
- Cosentino et al. “Remodeled eX vivo muscle engineered tissue improves heart function after chronic myocardial ischemia” Scientific Reports (2023) DOI: 10.1038/s41598-023-37553-8
- Ruud et al. “Regulation of cardiomyocyte t-tubule structure by preload and afterload: Roles in cardiac compensation and decompensation” The Journal of Physiology (2024) DOI: 10.1113/JP284566
- Llewellyn, Michael E. et al. “Orderly recruitment of motor units under optical control in vivo.” Nature Medicine (2010) DOI: 10.1038/nm.2228
- Ma et al. “Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle” Internation Journal of Molecular Sciences (2018) DOI: 10.3390/ijms19092643
- Hamoudi et al. “Muscle weakness and selective muscle atrophy in osteoprotegerin-deficient mice” Human Molecular Genetics (2020) DOI: 10.1093/hmg/ddz312
- Delfinis et al. “Muscle weakness and mitochondrial stress occur before severe metastasis in a novel mouse model of ovarian cancer cachexia” Molecular Metabolism (2024) DOI: 10.1016/j.molmet.2024
- Tatarenko et al. “Multiscale analysis of Klf10’s impact on the passive mechanical properties of murine skeletal muscle” Journal of the Mechanical Behavior of Biomedical Materials (2024) DOI: 10.1016/j.jmbbm.2023.106298
Related Products

605A: Dynamic Muscle Data Acquisition and Analysis System
Precise, custom software designed for real-time data acquisition, instrument control and data analysis with unique, integrative features. Includes force and SL control capability
Learn More
701C: Electrical Stimulator
Our high-powered, bi-phase stimulator designed for muscle researchers conducting the most demanding field stimulation protocols.
Learn More
800C: in-vitro Muscle Apparatus
Flexible design makes these systems the ideal choice for measuring muscle properties of intact muscle tissue in mice and rats
Learn More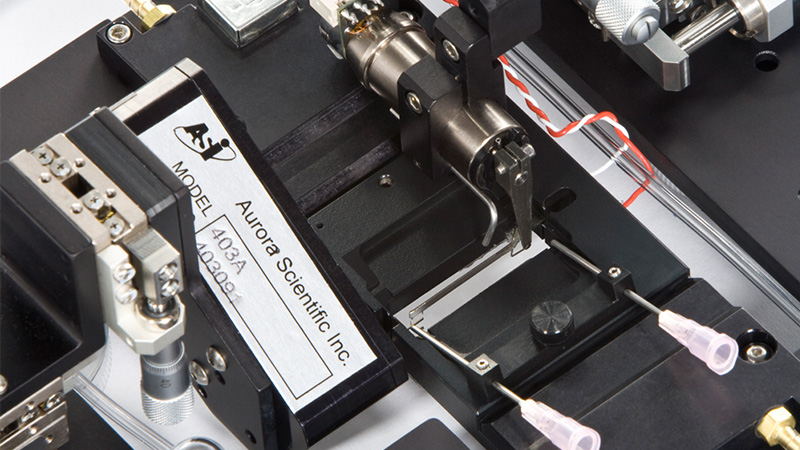
801C: Small Intact Muscle Apparatus – Microscope Mountable
Highly integrated apparatus, optimally designed to test small, intact muscle contractility
Learn More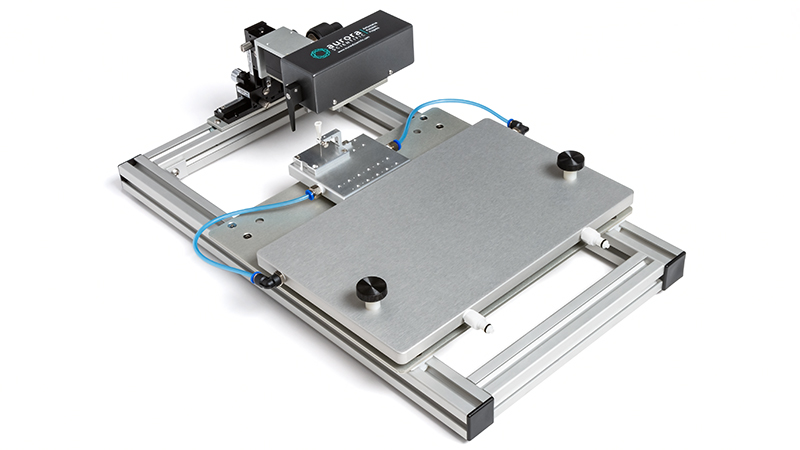
806D: in-situ Rat Apparatus
A beautifully designed, innovative apparatus for measuring in vitro, in situ and in vivo muscle properties in rats
Learn More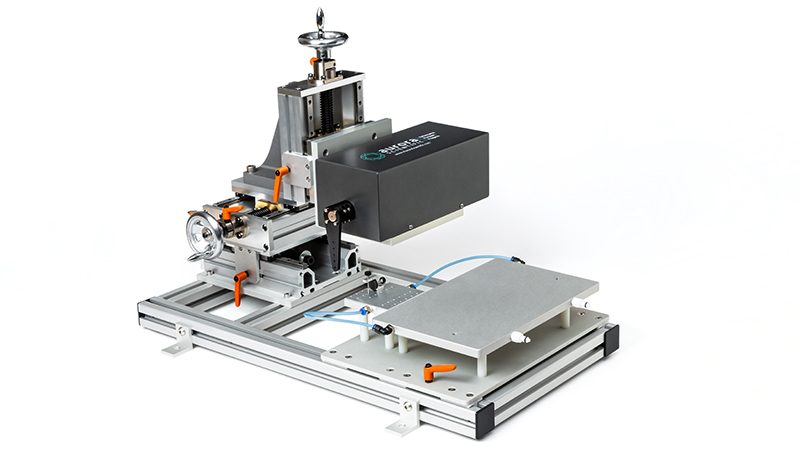
807B: in-situ Large Rodent/Small Animal Apparatus
Easily test in situ muscle tissue dynamics in large rodents, rabbits and other small animals
Learn More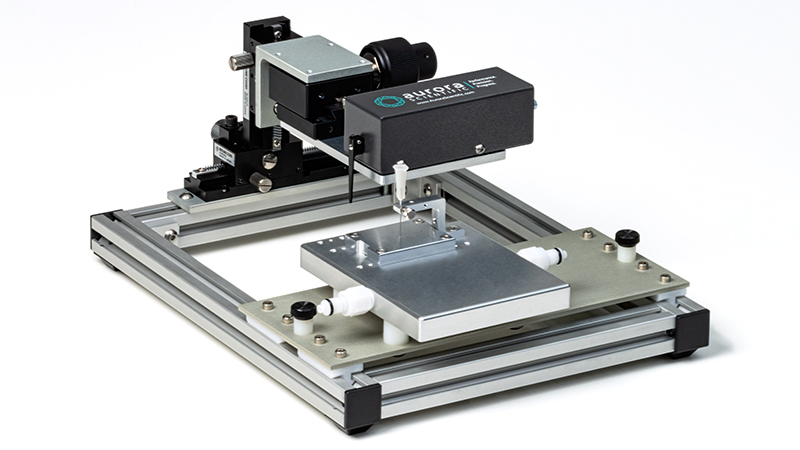
809C: in-situ Mouse Apparatus
A beautifully designed, flexible apparatus for easily measuring in vitro, in situ and in vivo muscle properties in mice
Learn More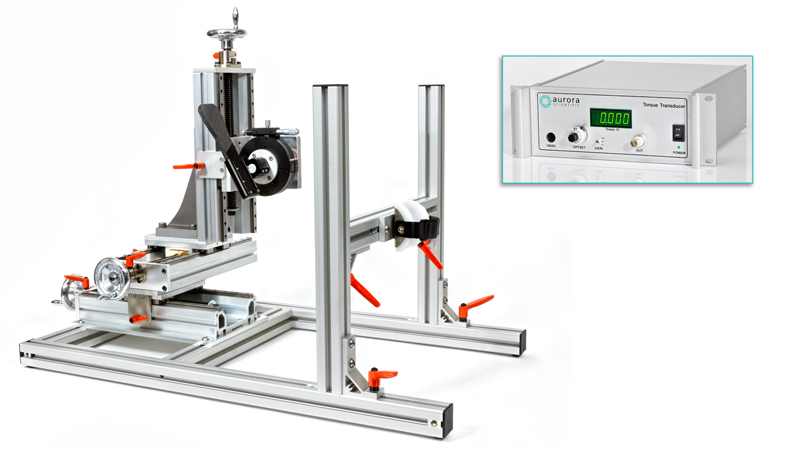
890A: in-situ Large Animal Apparatus
Easily test in situ muscle tissue dynamics in dogs, sheep, pigs and other large animals
Learn MoreRelated Systems
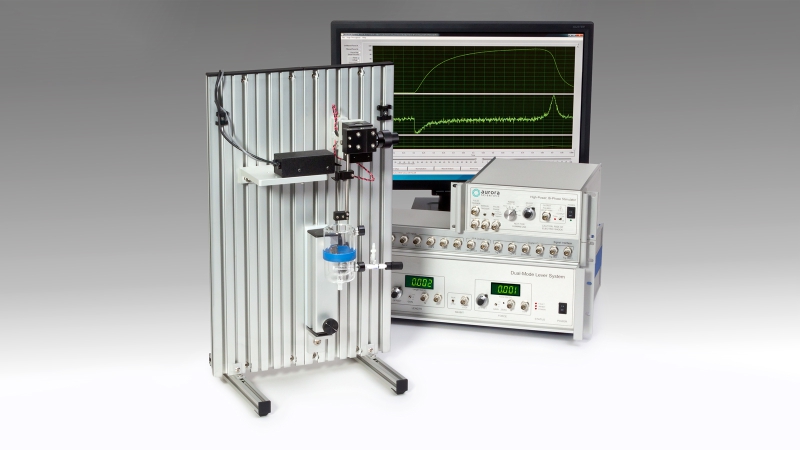
1205A: Isolated Muscle System for Rats
The 1205A isolated muscle test system is optimized for the study of mechanical properties of rat isolated muscle samples. Just like the 1200A, this system is ideal for a number of limb muscles such as TA, EDL, Soleus, Gastroc and for diaphragm strips and smaller muscle samples.
Learn MoreSpecifications
| Length Specifications | 300C | 300C-LR | 305C | 305C-LR | 309C | 310C | 310C-LR |
|---|---|---|---|---|---|---|---|
| Lever Arm Length [cm] | 3.0 | 3.0 | 4.0 | 4.0 | 6.0 | 8.0 | 8.0 |
| Length Excursion [mm] | 10.0 | 10.0 | 20.0 | 20.0 | 25.0 | 40.0 | 40.0 |
| Length Signal Resolution [micron] | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Length Scale Factor [mm/Volt ±2%] | 0.5 | 0.5 | 1.0 | 1.0 | 1.25 | 2.0 | 2.0 |
| Length Signal Linearity1 [%] | 99.5 | 99.5 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 |
| Length Step Response Time2 [millisecond] | 1.4 | 1.4 | 2.0 | 2.0 | 4.0 | 8.0 | 8.0 |
| Sinusoidal Frequency Response3 [Hz] | 450 | 450 | 300 | 300 | 135 | 90 | 90 |
1 = Over full length excursion; 2 = 1 to 99%, critically damped; 3 = -3dB point
| Force Specifications | 300C | 300C-LR | 305C | 305C-LR | 309C | 310C | 310C-LR |
|---|---|---|---|---|---|---|---|
| Maximum Force [N] | 0.5 | 1.0 | 5.0 | 10.0 | 20.0 | 50.0 | 100.0 |
| Force Signal Resolution [mN] | 0.5 | 0.5 | 1.0 | 2.0 | 4.0 | 10.0 | 20.0 |
| Force Scale Factor [mN/Volt ±2%] | 50 | 100 | 500 | 1,000 | 2,000 | 5,000 | 10,000 |
| Force Signal Linearity4 [%] | 99.5 | 98.5 | 99.8 | 99.8 | 99.8 | 99.8 | 99.8 |
| Force Step Response Time5 [millisecond] | 1.8 | 1.8 | 2.0 | 2.0 | 4.0 | 8.0 | 8.0 |
| System Friction6 [mN] | 0.9 | 0.9 | 3.0 | 3.0 | 8.0 | 20.0 | 20.0 |
4 = Over full length excursion; 5 = 1 to 99%, critically damped; 6 = Over full length excursion
| General Specifications | 300C | 300C-LR | 305C | 305C-LR | 309C | 310C | 310C-LR |
|---|---|---|---|---|---|---|---|
| Power Required | 100, 120, 220, 240 VAC, 50/60 Hz. available | ||||||
| Power Consumption [W] | 120 | 150 | 180 | 300 | 400 | 700 | 1,000 |
| Controller Weight [kg] | 8 | 8 | 8 | 8 | 10 | 10 | 18 |
| Motor Weight [kg] | 0.32 | 0.32 | 1 | 1 | 3.5 | 12 | 12 |
| Controller Dimensions [cm] | 48W (standard 19” rack mount) x 32D x 13H (3U) |
Explore Further

ULK2 is Essential For Degradation of Ubiquitinated Protein Aggregates and Homeostasis in Skeletal Muscle
The goal of the Lira Lab is to understand how autophagy and other proteolytic processes are regulated in skeletal muscle as they play distinct roles in maintaining skeletal muscle homeostasis, ...
Learn More
Applications of In Vivo Functional Testing of the Rat Tibialis Anterior for Evaluating Tissue Engineered Skeletal Muscle Repair
We describe an in vivo protocol to measure dorsiflexion of the foot following stimulation of the peroneal nerve and contraction of the anterior crural compartment of the rat ...
Learn More