Over the years, research into sarcopenia and aging has increasingly focused on improved models, mechanistic insights, and intervention testing. As researchers develop more sophisticated animal models, studies have shifted towards characterizing and reversing muscle loss with the goal of furthering treatment strategies. To highlight this trend, the following review covers new insights into the muscle manifestations of Hutchinson-Gilford progeria syndrome, the characterization of lingual dysfunction in rats, and the effect of a neuroprotective compound for reduced muscle weakness in aged mice.
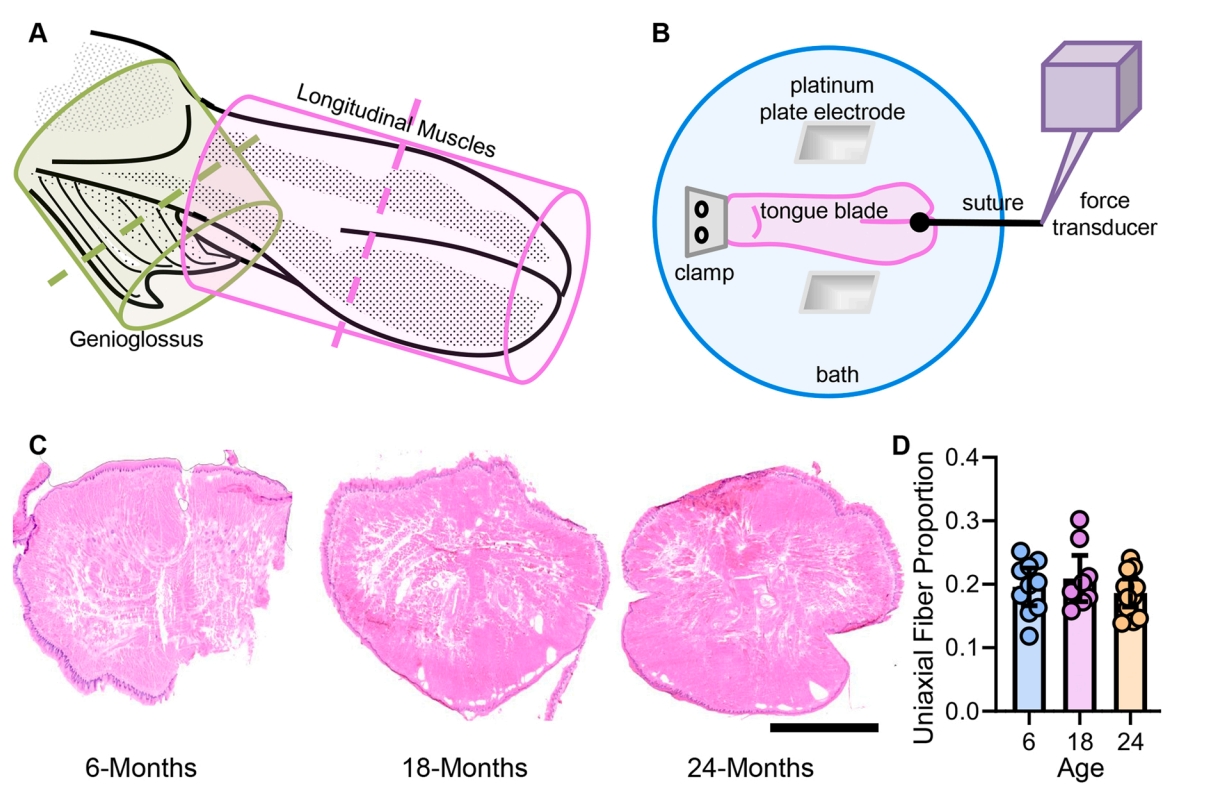
Featured image (Sieck et al. (2024), ©2023 Elsevier B.V. All rights reserved.) showing A) a schematic of the longitudinal (lavender) and genioglossus (green) muscles. The dashed line represents the plane of sectioning for CSA evaluations. B) Depicts the experimental setup for assessing longitudinal tongue muscle (lavender) uniaxial force, via stimulation in a tissue bath. C) H & E staining of a transverse section of the proximal region of the tongue blade, with the superior and inferior longitudinal muscles in cross-section. The scale bar represents 2500 µm. D) Scatterplot of the proportion of muscle fibers in the uniaxial direction of longitudinal muscle force across ages. Each symbol represents data from one rat.
Cardiac and skeletal muscle manifestations in the G608G mouse model of Hutchinson-Gilford progeria syndrome
Hutchinson-Gilford progeria syndrome (HGPS) is a rare, autosomal dominant disorder caused by mutations in the Lamin A gene (LMNA). Specifically, the point mutation c.1824C > T (p.G608G) leads to abnormal splicing of exon 11 and incorrect post-translational processing. This produces progerin, a truncated protein that accumulates in the nuclear envelope, causing abnormalities in nuclear morphology. Patients with the disorder typically present with alopecia, stiff joints, muscle weakness, and premature cardiac disease, often succumbing to myocardial infarction, heart failure, and stroke in their teenage years. Due to the rarity of HGPS, animal models such as humanized HGPS mice are a particularly powerful tool for studying the disease and exploring novel therapeutic agents. Hong et al. (2024) therefore sought to characterize the cardiac and skeletal muscle function of the G608G HGPS mouse model, as well as senescence-associated phenotypes in fibroblasts.
In addition to transthoracic echocardiography in the mice, they evaluated contractile function of both the TA and extensor hallucis muscles every 4 weeks. Using Aurora Scientific’s 300C Dual-Mode Muscle Lever System and 701C High-Power Stimulator, they stimulated the peroneal nerve for isometric contraction measurements. Contractile function of the anterior crural muscles was then assessed by measuring the isometric torque as a function of stimulation frequency (20–300 Hz).
Upon analysis, both cardiac and skeletal muscle perturbations were observed, with significant differences in weight between males and females. HGPS mice exhibited substantial weight loss, with a 30% reduction in males and 25% in females by 28 weeks. Cardiac function analysis revealed decreased cardiac output and stroke volume, with impaired diastolic function, but stable ejection fraction and fractional shortening. Structural cardiac changes included reduced end-diastolic diameter and increased left ventricular wall thickness, while skeletal muscle showed reduced muscle weight and size, decreased muscle torque, and increased fibrosis. Similar alterations were observed in HGPS fibroblasts, showing reduced proliferation, increased senescence markers, and upregulated SASP factors, which likely contribute to the observed muscle pathology. These findings highlight the profound impact of progerin on both cardiac and skeletal muscle and identify fibrosis and cellular senescence as key drivers of these manifestations.
Sarcopenia of the longitudinal tongue muscles in rats
Sarcopenia is characterized by the gradual loss of muscle mass and strength that typically occurs with aging. Decreased endurance, increased fatigue, weakness, and muscle wasting often underlie the condition, resulting in an overall reduction in physical function. In fact, decreased tongue strength in elderly individuals can even increase the risk of co-morbidities such as obstructive sleep apnea and pneumonia. While prior research in Fischer 344 (F344) rat models has shown that aging affects ventilatory function and diaphragm muscle strength, how aging impacts the intrinsic longitudinal tongue muscles has yet to be explored. As such, Sieck et al. (2024) aimed to measure the mechanical, fatigue, and fiber type-specific properties of these tongue muscles in both male and female F344 rats.
To achieve this, the entire intrinsic tongue of 6-, 18-, and 24-month-old F344 rats was isolated, excised, and submerged in a chamber of Rees-Simpson’s solution with bubbled carbogen gas. The base of the tongue was securely clamped and pinned to the chamber bottom, and a suture was placed in the midline of the tongue, with the other end tied to Aurora Scientific’s 300C Dual-Mode Muscle Lever System. Platinum plate electrodes were placed on either side of the muscle and stimulated with the 701C High-Power Stimulator, followed by force and fatigue assessments.
Although the proportion of muscle fiber types contributing to tongue bulk remained stable across all mice ages, isometric specific force decreased significantly in 24-month-old rats, with a 33% reduction in maximum tetanic force compared to younger rats. Fatigue increased with age, as evidenced by a higher fatigue index in the oldest rats, but residual force after fatigue did not differ significantly across age groups. Additionally, while type IIx/IIb fiber cross-sectional area decreased by 27% in 24-month-old rats, type IIa fiber size remained consistent, and overall muscle fiber cross-sectional area also reduced with age. These findings underscore how sarcopenia impacts tongue muscles and provides a backdrop from which to explore the underlying causes of age-related lingual dysfunction.
Neuroprotective treatment with the nitrone compound OKN‑007 mitigates age‑related muscle weakness in aging Mice
Although sarcopenia is known to significantly impact the health and quality of life of elderly populations, there are limited treatments to mitigate the loss of muscle mass and function during aging. The need for these interventions are further reinforced by the identification of sarcopenia initiating factors, such as peripheral motor neuron impairments and the loss of muscle innervation. These factors have been shown to contribute to mitochondrial dysfunction and elevated oxidative stress in muscle, spotlighting an untapped treatment avenue from which to test potential interventions. To explore this, Xu et al. (2024) evaluated the effects of OKN-007, an antioxidant and anti-inflammatory compound reported to reduce α motor neuron loss, on the muscle mass and function of aging mice.
The hindlimb extensor digitorum longus (EDL) muscle was isolated due to its well-documented response to oxidative stress in aging and other muscle diseases. Muscles were tied to the 300C Dual-Mode Muscle Lever of Aurora Scientific’s 1200A Isolated Muscle System and fixed in the water bath containing Krebs–Ringer solution. The 701C High-Power Stimulator was used to apply stimulation with flanking platinum plate field stimulus electrodes. Twitch, force frequency, and fatigue measurements were then recorded and analyzed using 615A Dynamic Muscle Control and Analysis Software.
Remarkably, OKN-007 treatment demonstrated promising effects in preserving age-associated muscle innervation and strength. While aged control mice experienced a 10% decrease in lean mass compared to middle-aged controls, the treatment effectively restored lean mass to middle-aged levels. Additionally, treated mice showed a notable improvement in EDL force production without any significant changes in muscle mass. On a molecular level, neuromuscular junction (NMJ) morphology improved with reduced denervation in treated mice, although NMJ fragmentation and area remained unchanged. The treatment also significantly enhanced mitochondrial respiration without any observed alterations in sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) activity, indicating a positive impact on mitochondrial function. These findings suggest that OKN-007 mitigates age-related muscle decline by enhancing muscle function and mitochondrial health, highlighting its potential as a neuroprotective intervention for sarcopenia.
Conclusions
Aging and sarcopenia research is essential for improving the health outcomes and quality of life for affected populations. By characterizing the musculoskeletal phenotypes in both HGPS and aged animal models, and testing the effect of a potential neuroprotective intervention, these studies by Hong et al. (2024), Sieck et al. (2024), and Xu et al. (2024) work in concert to drive promising advancements in the field.