Beyond the increasingly diverse list of animal models used to to study the link between genotype and phenotype, clever strategies to predict and validate structure-function relationships lies at the heart of comparative physiology. In the two articles that follow, the authors present innovative methods and models to update our understanding of structure & function.
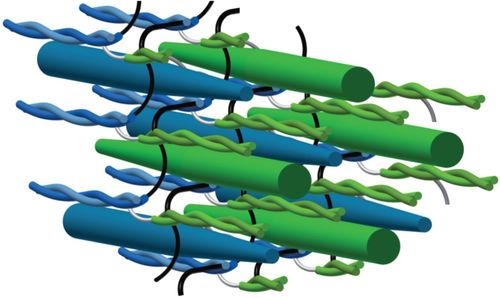
*Featured image from “Myosin filament sliding through the Z-disc relates striated muscle fibre structure to function”
Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle
X-ray diffraction is a powerful but underutilized tool to study muscle physiology. In concert with a servo motor/force transducer, it can be used to visualize myosin layer lines indicating whether myosin is active or relaxed in real time on the nanometer scale. This is particularly appealing for phenotyping – you could, for example, assess how mutations to sarcomeric proteins affect striated muscle function during contraction.
This is precisely what the authors of this publication set out to do, providing a detailed description of their protocol to demonstrate how other researchers can use x-ray diffraction to investigate structural changes in the sarcomere (e.g. due to a drug or genotype) and their effects on phenotype.
In the paper, the authors use blebbistatin (a myosin-II inhibitor which stabilizes the super-relaxed state of myofilaments) to study the structure and function of myosin heads at different states of relaxation. They dissected and mounted murine soleus and EDL muscle on the Aurora Scientific 300C-LR dual-mode muscle lever system, either in Ringer’s solution or blebbistatin+Ringer’s solution. They took x-ray diffraction measurements every 10 minutes, until force dropped below 5% maximum isometric tension.
The authors report that this method yields high-quality x-ray diffraction patterns,
“establishing the mouse intact skeletal muscle X-ray preparation as a potentially powerful tool to test structural hypotheses in health and disease.”
By comparing the diffraction patterns of samples with or without blebbistatin, they found that, in addition to the super relaxed state, there is at least one other helically-ordered configuration of myosin heads.
If you’re interested in learning more about their methodologies, the authors published a video with JoVE demonstrating their protocols for sample preparation, data collection and analysis. See:
Ma, W., & Irving, T. C. (2019). X-ray Diffraction of Intact Murine Skeletal Muscle as a Tool for Studying the Structural Basis of Muscle Disease. Journal of visualized experiments: JoVE, (149). doi: 10.3791/59559
Myosin filament sliding through the Z-disc relates striated muscle fibre structure to function
The isometric Force-Length Relationship (FLR) is a fundamental concept in muscle physiology. At short to intermediate muscle lengths, force increases proportionally with the length. Beyond the resting length, force decreases. At short lengths, muscle behavior historically has been thought of as a compressed spring, where less actin-myosin overlap translates to a reduced capacity to generate force. However, experimental findings conflict with this explanation. Unlike springs, myofilaments are too stiff to compress or fold at the Z-disc, and do not restore their resting length following contractions to short lengths. Essentially, there has not been a model that accurately predicts behavior across the entire FLR.In this paper, the authors present a model of muscle contraction that proposes that myosin filaments slide through (rather than fold or compress at) the Z-disc. They write,
“In our model, the transition from hexagonal to tetragonal actin filament arrangement near the Z-disc together with a thoughtful titin arrangement enables myosin filament sliding through the Z-disc. This sliding leads to swivelled cross-bridges in the adjacent half-sarcomere that dampen contraction”.
Essentially, the authors propose a mechanism by which myosin-actin myofilaments interact such that swivelled cross-bridges (not compressed or folded myofilaments) attenuate contraction at short lengths.
Their model reconciles the classic sliding filament and cross-bridge theories with experimental data, including some that has previously been difficult to explain. Using the Aurora Scientific 1400A Permeabilized Fiber System, the investigators showed that significant force could be generated at lengths shorter than classic zero force, and that fibers do not restore their resting lengths after contractions with an extended duration of activation. While a model based on spring-like myosin filaments cannot explain these phenomena, their model does.
As is typical for biological systems, the structure of muscle fibres gives rise to their function. However, the mechanisms by which this happens are often unclear or misunderstood. But with updated models and innovative methodologies to unravel the myriad of interactions between microstructures, we can improve our understanding of the molecular basis separating function and dysfunction.