Although great developments have been made in neuromuscular research, many mechanisms responsible for neuromuscular degeneration remain poorly defined and understood. This publication review highlights how several researchers have recently employed our instruments and technologies to better understand these mechanisms and uncover new therapeutic avenues for a variety of diseases and disorders.
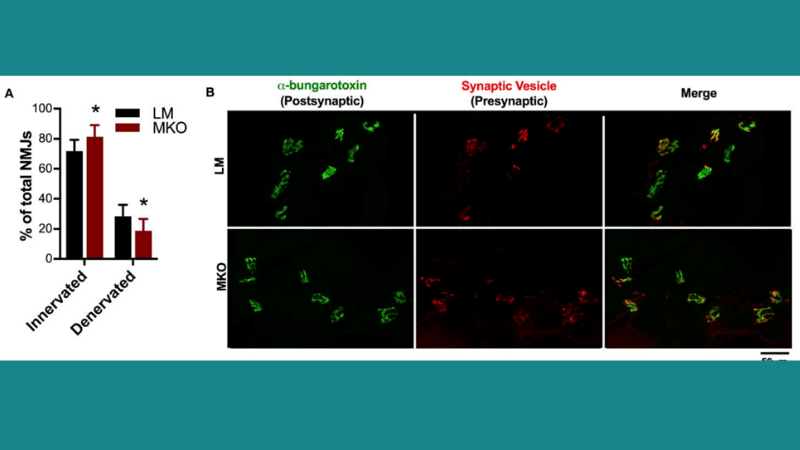
Featured image demonstrates that neuromuscular junction (NMJ) integrity changes with a lifelong Ulk1 deficiency (© 2021 Nichenko, A. S. et al.).
Increasing LRP4 Diminishes Neuromuscular Deficits in a Mouse Model of Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is a neuromuscular disease in which NMJ decline is observed. Since the underlying mechanism is poorly understood, Hui et al. explored how dystrophin deletion affects the NMJ and the agrin-LRP4-MuSK pathway, which is critical for NMJ maintenance. For this study, the authors made use of our 1300A Whole Animal System for in vivo twitch and tetanic force measurements in mice and found that increased LRP4 improves NMJ function and structure. Reduced LRP4 protein levels were observed the muscles of both DMD patients and model mice. LRP4 expression was also found to mitigate NMJ fragmentation and denervation, and increase MuSK activity and dystrophin-associated glycoprotein expression. The authors noted that the mechanism by which dystrophin regulates LRP4 expression must be studied in the future, and concluded that agrin signalling may serve as a novel therapeutic strategy against DMD.
Lifelong Ulk1-Mediated Autophagy Deficiency in Muscle Induces Mitochondrial Dysfunction and Contractile Weakness
The autophagy-related kinase Ulk1 may contribute to autophagosome degradation, but its role in healthy muscle aging is unclear. Nichenko et al. therefore assessed mouse muscle function in vivo and in vitro using our 300C-LR and 300B-LR dual-mode lever systems, respectively, to investigate the role of Ulk1-mediated autophagy in skeletal muscle aging. Results from this study suggest that Ulk1 maintains mitochondrial integrity through autophagosome assembly and degradation. Specific peak-isometric torque of the ankle dorsiflexors and specific force of the fast-twitch extensor digitorum longus muscles were reduced in muscle-specific Ulk1 knockout (MKO) mice compared to control mice, and greater mitochondrial content was observed in the MKO mice. Furthermore, Ulk1 phosphorylation to total Ulk1 protein content was reduced in aged muscles in both mice and humans, which may contribute to autophagosome degradation and dysfunctional mitochondrial accumulation. The authors concluded that Ulk1 may provide a therapeutic target for maintaining muscle quality throughout life.
Regulation of Excitation-Contraction Coupling at the Drosophila Neuromuscular Junction
Although the Drosophila neuromuscular system is widely used to characterize synaptic development and function, little is known about how specific synaptic changes affect neuromuscular transduction and muscle contractility. Ormerod et al. have therefore examined excitation-contraction coupling (ECC) at Drosophila larval NMJs using our high resolution 403A force transducer system along with our 615A Dynamic Muscle Control software for data acquisition. The key findings of this study indicate that the ECC machinery in Drosophila larvae provides a valuable system for examining the components that contribute to and regulate ECC neuromotor circuitry. The authors reported that larval muscle contraction force increases with motoneuron stimulation frequency and duration, and also determined that temperature is critical in muscle contractility. Genetic and pharmacological manipulations were also found to affect muscle contraction strength and duration. Lastly, the authors identified an FMRFa peptide that drastically increases muscle performance and noted that their future research will investigate its ability to recruit neurons and other effectors.