Featured Image courtesy of J.R. Huot et al. (2021).
Chemotherapy can increase the survival rate of patients with common cancers, but often causes severe side effects. These side effects can limit the use of chemotherapy by oncologists, since they can result in debilitating sensory disorders and neuropathies that reduce quality of life for many patients and can persist for months or years after discontinuing chemotherapy. Cachexia, skeletal muscle weakness and atrophy caused by cancer and its treatments, drastically impairs quality of life and worsens survival outcomes in cancer patients. Since there are currently no approved treatments for cachexia, further investigation into the causes of cachexia induced by cancer and chemotherapy is warranted. This publication review will address two articles that investigate the effects of chemotherapy and cancer on skeletal muscle in mice and rats.
Cancer Exacerbates Chemotherapy-Induced Sensory Neuropathy
In this article, S.N. Housley, et al. (2020) present the first evidence that long-lasting sensory neuropathy experienced by cancer patients is likely dependent on interactions between chemotherapy and the cancer itself, not just as a result of their cancer treatments. They tested their hypothesis by uncoupling the independent and combined effects of cancer and chemotherapy on sensory neurons in a rat model of colorectal cancer. To record spiking activity from mechanosensory neurons of the triceps surae muscles (lateral and medial gastrocnemii and soleus), our 305B-LR Dual Mode Muscle Lever was used to subject the muscle to physiologically relevant mechanical stimuli via stretching. The results of their study demonstrated a negative functional interaction between cancer and chemotherapy in sensory neurons in vivo. They also indicated that signaling deficits induced by chemotherapy or cancer alone were inadequate to reproduce the extent, direction and quantity of dysfunctions compared to those exhibited in both cancer and chemotherapy combined. Understanding these cancer-chemotherapy interactions and their effects on potential cancer treatments is both challenging and necessary for developing effective therapies in the future.
Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity
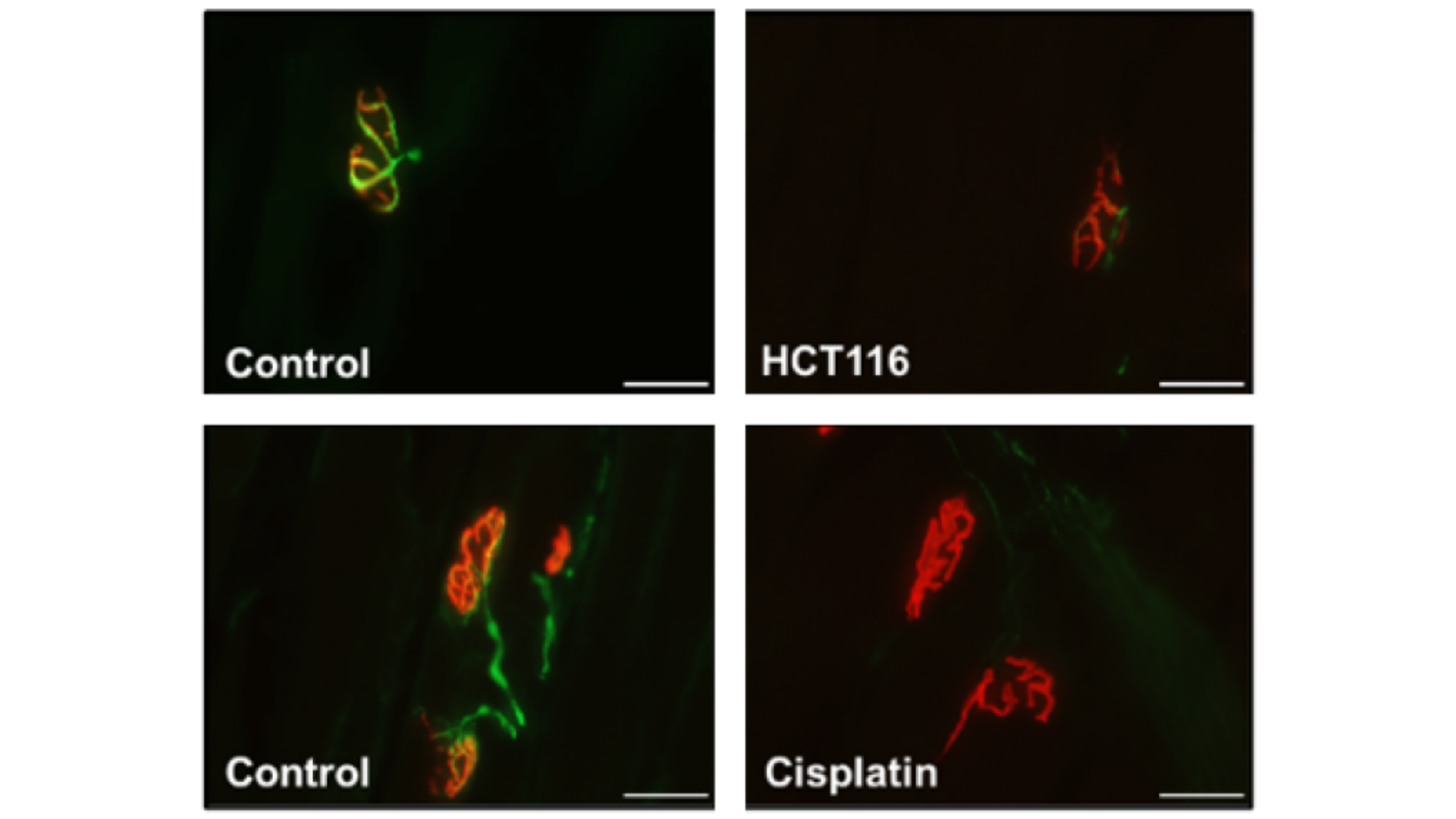
In this article, J.R. Huot et al. (2021) sought to examine skeletal muscle wasting, weakness and loss of motor unit function in male mice hosting cancers or being administered chemotherapeutics. Mice bearing colorectal cancers and mice receiving the folfiri and cisplatin (chemotherapeutics) were assessed for in vivo and ex vivo muscle force, and for in vivo electrophysiological indices of motor unit connectivity. The extensor digitorum longus muscle contractility was assessed using our ____ and force data was analyzed using our 615A: Dynamic Muscle Control and Analysis Software. In vivo and ex vivo contractile force were reduced in male mice with colorectal cancers, and in male mice receiving chemotherapy compared to their respective experimental controls. Motor unit number estimation was also reduced in the cancer-positive and chemotherapy-treated mice, and neuromuscular junction protein expression suggested that cancer and chemotherapy both significantly alter innervation of these muscles. These results reveal that the loss of innervation, and thus motor unit connectivity, may contribute to worsened cachexia and that these parameters should be considered in future studies involving models of chemotherapy- and cancer-induced muscle wasting and weakness.