In scientific experimentation, the products available do not always fit the model perfectly. Many labs build their own devices or make modifications to existing products to make their experiments easier, more accurate, or more reproducible. In this publication review, we highlight two groups that have done just that! They have taken our 1300A Whole Animal System for Mice with a 305C-FP Dual-Mode Footplate and customized it to fit their research needs and provide novel approaches to experimental measurements. Cheers to furthering muscle and skeletal mechanics research and the scientists who are implementing custom modifications to our equipment to make it happen!
In vivo Measurement of Knee Extensor Muscle Function in Mice
Consistent and reproducible methods for repeatedly and non-invasively studying knee extensor strength in rodents are relatively limited. Previously, a method to measure rat quadriceps muscle contractility was developed, however it requires extensive customization of equipment that is not readily available to most scientists.
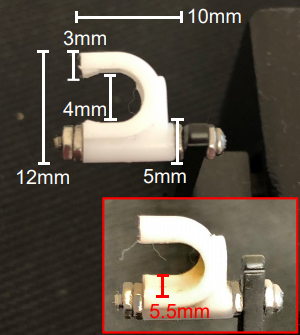
In this article, Brightwell et al. (2021) have demonstrated a novel method for non-invasively measuring knee extensor strength in rodents. This method allows for repeated measures in the same mouse over time using commercially available equipment with minor modification.
The minor modification of this system is the addition of the adjustable plastic piece used to hold the lower hind limb at the anterior tibia seen here on the right.
This modification facilitates the reproducibility of this method among different labs, and provides a more direct comparison to human strength outcomes. The measurement and analysis of muscle function in rodents is important to inform translational research on skeletal muscle adaptations observed with disease and therapeutic treatments.
To see this novel method in action, click here to view this publication on the Journal of Visualized Experiments website.
Disparate bone anabolic cues activate bone formation by regulating the rapid lysosomal degradation of sclerostin protein
Sclerostin is a fundamentally important osteocyte-derived protein that inhibits bone formation. The downregulation of sclerostin (gene name Sost) in osteocytes mediates bone formation in response to mechanical and hormonal cues.
In this publication, Gould et al. (2021) use a mouse forelimb mechanical loading model to assess bone strains generated by ulnar loading and isolate proteins from these upper limbs for Western blotting analysis. Their data suggest that sclerostin abundance is regulated via post-translational modification in osteocytes to regulate bone formation, and that post-translational modification tags the protein to be degraded by lysosomes. These results reveal that osteocytes respond to mechanical and hormonal cues by redirecting the sclerostin protein from a secretory pathway to lysosomes for rapid degradation.
To further this research, this group assessed lysosomal degradation of sclerostin in iPSC-derived osteoblasts from Gaucher disease patients. These osteoblasts had significantly increased levels of sclerostin compared to iPSC-derived osteoblasts from healthy patients without Gaucher disease. To confirm this degradation pathway, they treated Gaucher iPSC-derived osteoblasts with recombinant GCase, which restored lysosomal function, and this treatment restored sclerostin abundance to control levels.
This publication provides key insights into the unexpected, rapid regulation of bone formation by sclerostin and reveals a new therapeutic target that can be exploited to improve bone mass in conditions such as osteoporosis, Gaucher disease, and other bone-degradation related diseases.