Gearing up for the much-anticipated Society for Neuroscience (SfN) meeting, known as the premier global neuroscience event, this month’s publication review centers around nociception. In brief, nociception is defined as the neural process of encoding noxious stimuli – a field which has garnered significant interest in light of worldwide chronic pain prevalence. Here, we highlight how recent advances on the role of sensory neurons elucidate nociceptive pathways. Moreover, we cover how a novel nociceptive methodology provides a promising technique for standardizing and assessing nocifensive behaviours.
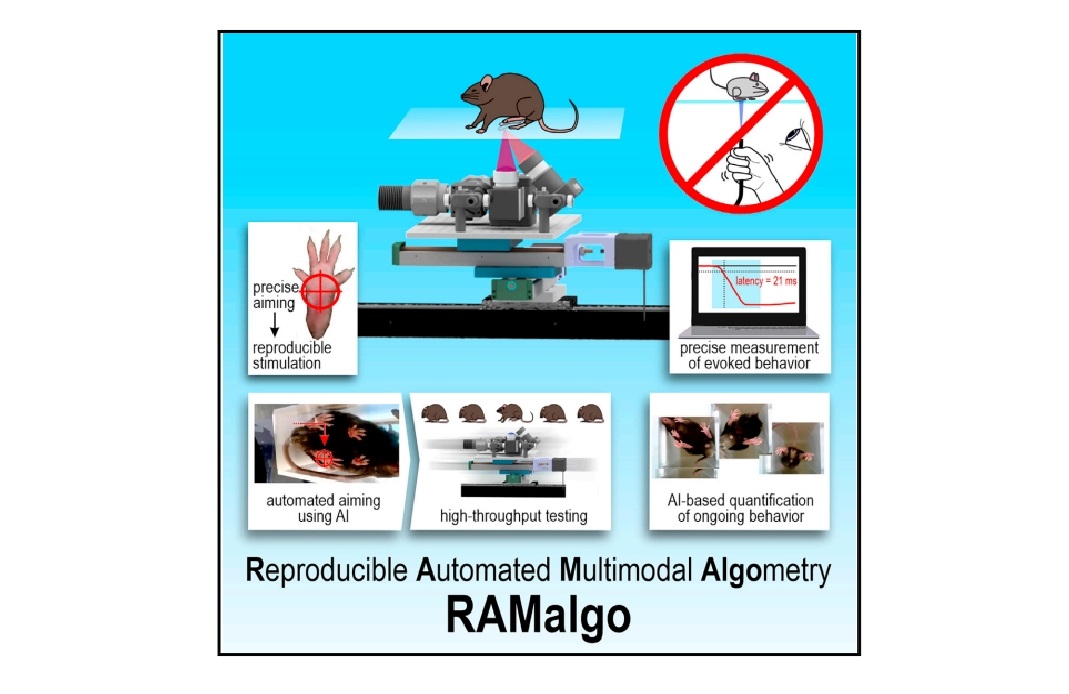
Featured image (©Dedek et al (2024), licensed under CC BY 4.0 DEED) providing an overview of the reproducible automated multimodal algometry (RAMalgo) system for improved preclinical pain testing. The system is capable of delivering consistent and measurable optogenetic, thermal, and mechanical (tactile) stimuli.
Distinct local and global functions of mouse Aβ low-threshold mechanoreceptors in mechanical nociception
Chronic pain, classified as pain persisting beyond 3 months, affects more than 30% of people around the globe. Among the many symptoms implicated in the disorder is mechanical hyperalgesia, defined as an increased pain triggered by mechanical stimuli. On the mechanistic level, specific types of sensory neurons, called Aβ low-threshold mechanoreceptors (LTMRs), are responsible for detecting mechanical stimuli. Despite great interest in these neurons, their role in transmitting mechanical hyperalgesia remains a topic of debate. Therefore, Gautam et al (2024) sought to elucidate the roles of Aβ-LTMRs in this regard, with the ultimate hope of expanding treatment avenues for mechanical hyperalgesia.
The study utilized various behavioral assays to investigate mechanical sensitivity and pain responses in mice, including Aurora Scientific’s 300C-I Dual-Mode Indenter. The indenter enabled precise delivery of controlled mechanical stimuli during single-fiber recordings from skin receptors, allowing the accurate determination of mechanical thresholds. In addition to the indenter, a wide range of assays, including texture-preference, plantar-pinch, and optogenetic tests, were performed to assess mice responses to different sensory challenges.
Results showed that global ablation of Aβ-LTMRs decreased sensitivity to light mechanical forces but increased baseline nociception and hyperalgesia in chronic pain models, suggesting a general inhibitory role. Conversely, local activation of Aβ-LTMRs induced pain responses, indicating that their local activity can promote hyperalgesia. Overall, Aβ-LTMRs appeared to play a dual role: the inhibition of mechanical nociception globally as well as the promotion of hyperalgesia locally. These findings underscore the need for treatment strategies that involve global activation and local inhibition of Aβ-LTMRs to effectively manage mechanical hyperalgesia.
Effects of inflammation on the properties of Nav1.8-ChR2-positive and Nav1.8-ChR2-negative afferent mechanoreceptors in the hindpaw glabrous skin of mice
Mechanoreceptors in the skin play a crucial role in sensing mechanical stimuli. Alterations to mechanoreceptors from pathological conditions, such as tissue inflammation, contribute to mechanical hyperalgesia and allodynia. Previous research has shown conflicting evidence regarding the sensitization of mechanoreceptors following inflammation, highlighting a need to clarify their functional changes. In this study, Yamada et al (2024) utilized Nav1.8-ChR2 mice to investigate the properties of different mechanoreceptors in the hindpaw glabrous skin, focusing on how these properties are affected by inflammation. The aim of the study was to examine the changes in mechanoreceptor properties following inflammation, particularly in the context of Nav1.8-ChR2-positive high-threshold mechanoreceptors (HTMRs) and Nav1.8-ChR2-negative low-threshold mechanoreceptors (LTMRs).
The researchers injected either saline or Complete Freund’s Adjuvant (CFA) into the hindpaws of Nav1.8-ChR2 mice and conducted behavioral assessments to evaluate pain sensitivity. They then performed ex vivo skin-nerve preparations for electrophysiological recordings, using Aurora Scientific’s 300C-I Dual-Mode Indenter to apply controlled mechanical stimuli. Light stimulation was also employed to differentiate between Nav1.8-ChR2-positive and negative mechanoreceptors.
Upon analysis, CFA-induced inflammation was found to significantly alter the mechanical thresholds of mechanoreceptors in Nav1.8-ChR2 mice. Specifically, many Nav1.8-ChR2-positive Aβ-fiber mechanoreceptors exhibited a reduced mechanical threshold, indicating increased sensitivity, while Nav1.8-ChR2-negative Aβ-fiber mechanoreceptors showed heightened thresholds. Mechanical indentation tests revealed that the lowest force eliciting action potentials in Nav1.8-ChR2-positive fibers was lower in the CFA group compared to the controls, suggesting enhanced mechanosensitivity in these fibers following inflammation. Conversely, the increased threshold in Nav1.8-ChR2-negative fibers highlighted a contrasting effect of inflammation on different mechanoreceptor types. These findings expand our understanding of the mechanisms behind mechanical allodynia and hyperalgesia in pathological conditions.
Reproducible and fully automated testing of nocifensive behavior in mice
Preclinical pain testing often suffers from poor reproducibility, low throughput, and an overemphasis on reflexive responses, leading to challenges in accurately assessing nocifensive behavior in mice. To address these issues, Dedek et al (2024) developed a fully automated pain-testing robot capable of delivering standardized optogenetic, thermal, and mechanical stimuli while precisely measuring withdrawal responses. This system, termed RAMalgo (reproducible automated multimodal algometry), utilized machine learning for automated aiming and data collection, enhancing the ability to analyze both evoked and non-evoked behaviors. With RAMalgo, the researchers sought to improve the standardization and comprehensiveness of pain testing, thereby facilitating more reliable and informative assessments of pain sensitivity in rodent models.
The study primarily used Ai32(RCL-ChR2(H134R)/EYFP) mice, selectively bred with different Cre lines to express channelrhodopsin-2 (ChR2) in distinct sensory afferents. Pain testing was conducted using Aurora Scientific’s 300C-I Dual-Mode Indenter for precise mechanical stimulation, while a custom photostimulator integrated red, blue, and infrared light sources to deliver controlled stimuli, allowing for automated measurement of withdrawal responses and comprehensive behaviour analysis via high-speed video and machine learning.
The findings demonstrated that RAMalgo effectively quantified nocifensive behaviours in mice, showing an inverse correlation between withdrawal latencies and optogenetic stimulation intensity. High-speed video analysis also revealed differences in paw withdrawal dynamics not captured by traditional methods. Additionally, the integration of machine learning facilitated accurate tracking of mouse movements and behaviours, enhancing the assessment of pain responses beyond reflexive actions. Overall, the study showcased RAMalgo’s significant improvements in reproducibility, throughput, and the comprehensiveness of pain assessments in preclinical research.
Conclusions
These studies by Gautam et al (2024), Yamada et al (2024), and Dedek et al (2024) collectively delineate the roles of specific mechanoreceptors in pain mechanisms, revealing insights into how they function under normal and pathological conditions. Taken together, these findings are critical for developing targeted therapies for nocifensive disorders and improving research methods in the field.