How the brain encodes information is an ongoing discussion in neuroscience, and a specific area of interest is how odors are mapped and programmed in mammalian brains.
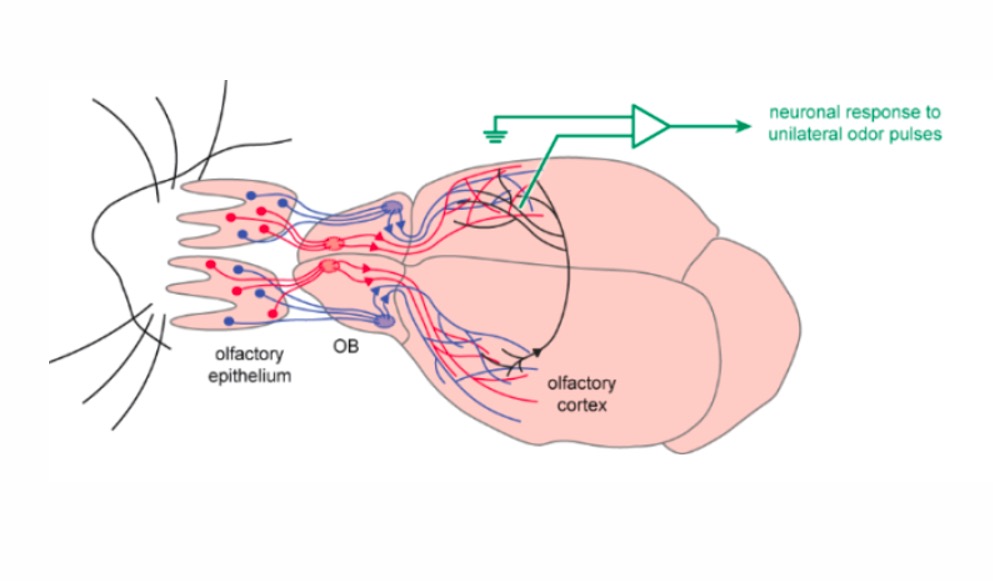
Odor detection starts with odorant molecules entering the nose and binding to olfactory receptors. These odorants will bind to a unique set of olfactory receptors based on the composition of the odor and these olfactory receptors may even overlap to produce different stimuli. In rodents, each olfactory sensory neuron expresses a single olfactory receptor and leads to one glomerulus. Each odorant molecule then activates a unique set of glomeruli, which in turn activates the olfactory bulb (OB). In mammalian brains, neurons that are spatially close to each other respond to cues that are also physically close to each other in their sensory space. While this principle is common in vision, hearing, pain perception, body position awareness and temperature perception, olfaction appears to be an exception. Olfaction encoding appears to be unstructured and an open question remains; how does a coherent representation of odorants arise in the olfactory cortex?
The following two publications explore olfactory encoding in mammals: Grimaud et al. use unilateral odor delivery in mice to explore the bilateral alignment of receptive fields, while Perl et al. test how temporal encoding affects odor perception in human participants.
Bilateral Alignment of Receptive Fields in the Olfactory Cortex Points to Non-Random Connectivity
In this study, the authors confronted the predominant view of OC connectivity by comparing the bilateral odor tuning of individual neurons in the olfactory cortex (OC) of mice. If neuronal projections between hemispheres of the brain are truly random, it is expected that there would be little to no relationship between the way the OC responds to ipsilateral and contralateral odor presentations.
A main goal of this study was to compare the bilateral odor receptive fields of OC neurons in conscious mice. To do so, the authors developed a new facial mask for unilateral odor delivery in awake mice. The mask was made of a large piece of tubing surrounding the mouse’s muzzle. On each side of the mask, a piece of tubing delivered odors to each nostril at a small flow rate. A photoionization detector (miniPID) was used to confirm that the mask could deliver similar amounts of odor on each side of the mask.
These authors found that odor responses of individual neurons in both nostrils closely matched and that odor identification was optimized when information arrived from one nostril and transferred to the other nostril. Analysis showed that such coordinated odor tuning is not compatible with random connections. These results show that odor information across the two hemispheres is highly coordinated, despite the distributed nature of the sensory representations in the OC.
The contribution of temporal coding to odor coding and odor perception in humans
In this study, the authors examined whether glomerular activation times affect odor perception in humans. Using temporal odor sequences, they manipulated temporal features that are involved in odor coding and measured their effect on odor perception during discrimination and odor-rating tasks. They tested whether humans could discriminate between a pair of temporal odor mixtures (TOMs) composed of the same two components delivered in rapid succession in either one temporal order or its reverse. The TOMs should theoretically activate the same olfactory neurons, but at different times, and thus differ mainly in the timing of neuronal activation.
They found that most participants could hardly discriminate between TOMs, although they easily discriminated between a TOM and one of its single component odors. However, when participants were notified of the successive nature of the odor components in advance, they succeeded in discriminating between the TOMs. Odor delivery and clearance were verified to be precise across trials with a mini photoionization detector (200B miniPID). These results suggest that the timing of glomeruli activation can be subjugated to extract odor-related information, although it does not change the odor perception substantially.