The maintenance of muscle mass is critical in the fight against cancer. Reduced muscle function is associated with a poorer prognosis and can result in the loss of independence, to the great detriment of patients and their quality of life. Unfortunately maintaining body mass can be difficult due to cachexia, a common side effect of cancer. Cachexia is a wasting syndrome characterized by weakness, loss of appetite, fatigue and weight loss.
In the pursuit of better treatments for cancer cachexia, rodent models are invaluable. In these animals, assays of muscle function are fundamental to evaluate the disease state, its progression and how modulating various factors translates into changes in function. In the publications below, the authors highlight 3 different assays you can use to assess muscle function in rodent models of cancer cachexia.
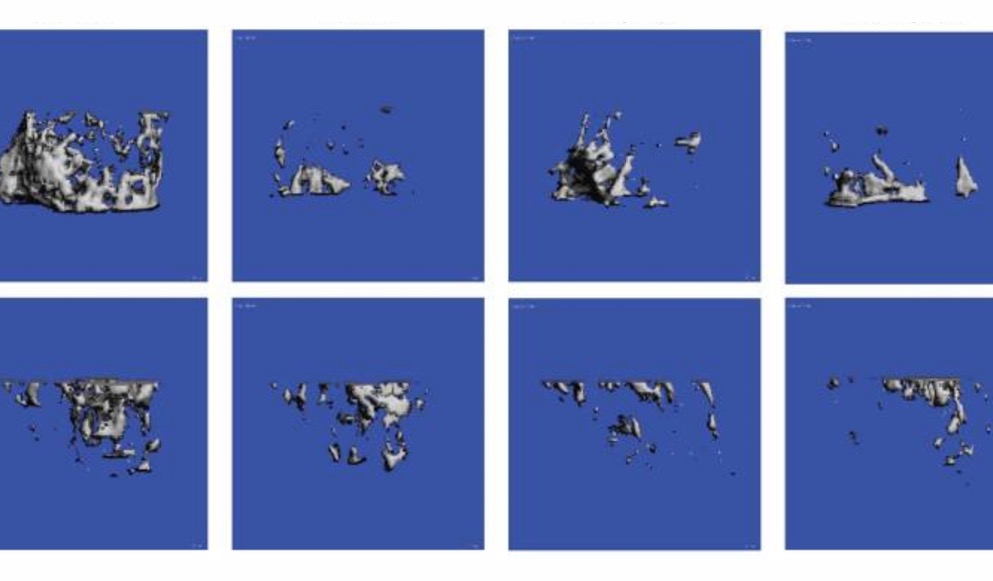
Featured Image courtesy of Brian Hain et al.
Chemotherapy‐induced loss of bone and muscle mass in a mouse model of breast cancer bone metastases and cachexia
While there’s no doubt that chemotherapy can be an effective treatment for many kinds of cancer, it often carries significant risk of side effects. One such side effect is the loss of bone mass. And because skeletal muscle is highly dependent on bone to maintain strength (and vice versa), the authors were interested in studying how chemotherapy affects the musculosketal system.
They treated both healthy mice and nude mice inoculated with human breast cancer cells with carboplatin, a chemotherapy drug that interferes with DNA replication resulting in cell death. To assess the contractile properties of the muscle, they dissected the EDL and mounted it on an Aurora Scientific isolated muscle bath system to measure ex vivo force production. Compared to control mice, EDL from mice treated with carboplatin had reduced force production, whether or not it also had cancer. In addition, the authors point out that carboplatin did not mitigate muscle weakness due to cancer, but it did not exacerbate it, either.
These results highlight the fact that while carboplatin treatment provides an important therapeutic benefit, it does have significant negative side effects which must be considered as part of cancer treatment.
PERK regulates skeletal muscle mass and contractile function in adult mice
Effective management of misfolded proteins is critical for the development of skeletal muscle. If misfolded proteins build up in the endoplasmic reticulum, Protein kinase R-like endoplasmic reticulum kinase (PERK) helps to activate the unfolded protein response (UPR), which directs the cell towards apoptosis. Previous research has shown that PERK is activated in several animal models of cancer cachexia, but its role in the reduction of muscle mass is unknown. In this study, Yann Gallot investigated how deletion of PERK affects the regulation of muscle mass.
Previous studies have shown that germline deletion of PERK is lethal, so they use Cre-Lox recombination to generate skeletal muscle-specific PERK-knockout mice. At 12 weeks, the mice were given tamoxifen, inducing the deletion of PERK. 4 weeks after deletion, they measured force production using the Aurora Scientific 1300A. After anesthetization, plantarflexion was stimulated at several different frequencies to measure specific twitch force and isometric tetanic force in vivo. Compared to control mice, there was no significant difference in specific twitch force between control and PERK-KO mice. At low stimulation frequency, there was also no significant difference for tetanic force. However, at higher frequencies tetanic force from PERK-KO muscle was significantly reduced.
These results suggest that PERK is critical for the stable force production. In the knockout mice, atrophy was apparent after 4 weeks and muscle fatigued more quickly. The authors note that this study provides initial evidence that PERK is necessary for the regulation and maintenance of muscle mass, but more research will be necessary to understand whether the activation of PERK can be modulated to mitigate muscle loss, for example due to cancer cachexia.
Skeletal muscle function during the progression of cancer cachexia in the male ApcMin/+ mouse
Despite steady research efforts in the study of muscle mass loss due to cancer cachexia, the authors note that,
“the drivers of cancer-induced muscle functional decrements are only beginning to be understood.”
In this paper, Brandon VanderVeen et al. tracked progression of cancer cachexia in the ApcMin/+ mouse model, looking at functional measures alongside inflammatory factors suspected to play a role in cachexia.
They evaluated body mass, muscle mass, twitch properties, force production, fatiguability, protein and mRNA expression. Similarly to the previous article, they studied contractile properties in situ. For contractile experiments, authors isolated the tibialis anterior, exposed the sciatic nerve and secured the distal tendon to an Aurora Scientific dual-mode lever. Notably, they found that progressive reduction of muscle function in the mouse model is strongly associated with inflammatory signaling. In addition, they reported the novel finding that fatiguability was significantly increased before the development of cachexia.
The authors note that several clinical trials for drugs to treat cachexia have failed due to a lack of functional improvement. The development of effective therapeutics is very dependent on our understanding of physiological function, so the finding that cachexia is associated with inflammatory signaling may be a valuable line for future research to follow.