This blog summarizes the discussion in our first webinar entitled “Three Techniques, One System: How to Effectively Characterize Complete Muscle Function“. It is intended to give a quick overview of the topics discussed and to be a primer for our 1300A 3-in-1 Whole Animal System for measuring muscle function in animal models.
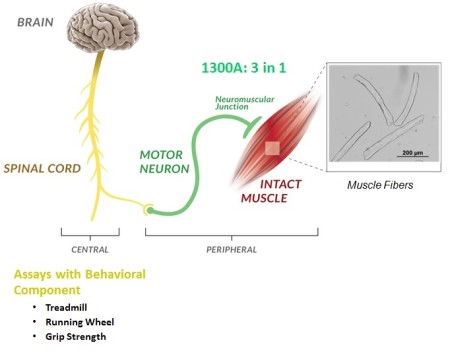
An important goal of many researchers is assessing an animal model’s physical performance. While there are many different informative screening assays used in research, few are able to accurately describe functional phenotypes that are specific to neuromuscular function. This is mainly due to the behavioural and biological variables that arise within sample groups. To accurately determine the neuromuscular function of a model animal, we require an assay that specifically tests the strength of muscles relative to their cross-sectional area or the masses of the animals themselves. On top of that, the voluntary component of the assay must also be removed.
Figure Courtesy of Ward & Khairallah
Aurora Scientific’s solution to this problem is our 1300A 3-in-1 Whole Animal Muscle System. The 3-in-1 is based around our 300C Dual-Mode Muscle Lever series and provides flexible and accurate measurement of muscle function and properties in situ, in-vivo and in-vitro.
Using the dual-mode lever allows both the force and length of the sample to be measured and altered during an experiment. The system is able to control one of these parameters while measuring the other and to switch instantaneously between the two settings. Since only one point of attachment to the muscle is needed, it enables the researcher to have a very sophisticated experimental design using complex protocols that can accurately simulate physiological conditions. As the conversion between the three set ups can be completed with ease, this gives the researcher greater experimental versatility. Because of this, the 1300A has a very diverse group of users:
- muscle physiologists
- geneticists
- neuroscientists
- pharmacologists and biochemists
- exercise scientists
- metabolic and cardiovascular scientists
- bioengineers and biologists
How to effectively characterize complete muscle function
in-vitro
Photo Courtesy of Wilkinson Laboratory, SJSU
When using the 1300A for in-vitro studies, the muscle of interest is surgically removed and placed in an oxygenated and temperature controlled bath to maintain viability. One tendon is anchored to a fixation clamp, while the other tendon is sutured onto the lever arm of the dual-mode lever. The muscle is then stimulated by field electrodes fixed inside the bath, however, if the nerve is still present suction electrodes may also be used.
This experimental design can be used for any small muscle whose metabolic needs can be met through diffusion. These include:
- EDL
- soleus
- lumbricalis
- diaphragm (strips)
- heart muscle (papillary and trabeculae)
This method is preferable for samples from younger animals as the increased muscle size of older animals can be quite limiting to diffusion.
Strengths
- surgical excision allows for direct measurement of muscle function (contractility) independent of neuronal integrity and other variables
- allows for in-vitro nerve-muscle function to be assessed
- chemical/pharmacological manipulation possible – independent of oral and bio-availabilty
Challenges
- blood flow negated
- technically challenging
- prep viability limited by O2/metabolic/CO2 diffusion in and out of inner muscle fibers
- temporal repeated measures impossible
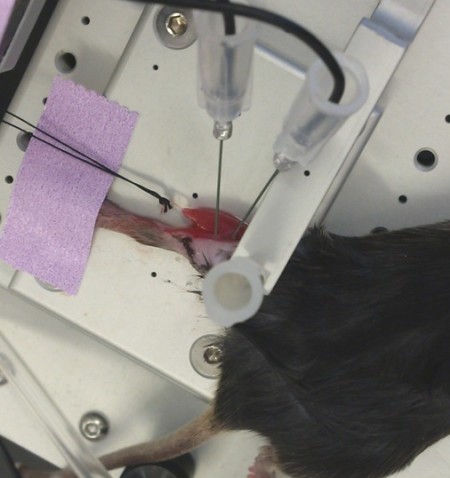
in-situ
Photo Courtesy of Granzier Laboratory, University of Arizona
This method can be used to measure force directly from any muscle that is not highly pennate and that can be ligated at one end while the opposite remains intact.
The main muscles studied are usually:
- TA
- EDL
- gastrocnemius
- soleus
However, muscles like the trapezius and masseter are also viable for this method of assessment.
In-situ is similar to the in-vitro set up since a single muscle is still isolated, however, there is less surgical involvement. The animal is terminally anaesthetized and a small incision is made in the animal’s leg to expose the muscle of interest. The skin is retracted as least halfway up the leg in order to expose the muscle group. The distal tendon is then severed and sutured onto the dual-mode lever arm. The exposed tissue can be kept moist with a saline or mineral oil drip. The muscle is then stimulated by needle electrodes placed directly on the corresponding nerve or by resting the electrodes directly on the muscle. This allows for force to be measured from a single muscle while its metabolic needs are still met by its own blood supply.
Strengths
- neuromuscular blood supply is left intact despite surgical invasion
- direct measurement of a specific muscle’s contribution
- determination of muscle specific contribution to function is possible by direct muscle stimulation
Challenges
- invasive and technically difficult
- temporal repeated measurements are challenging
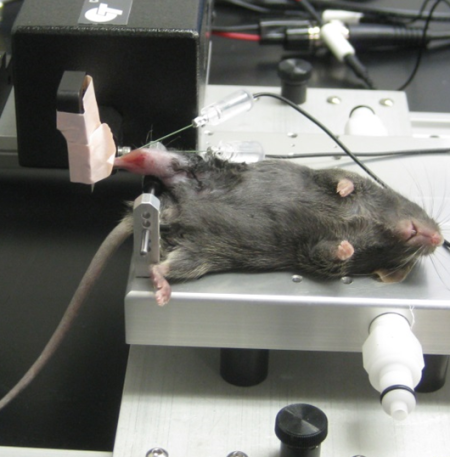
in-vivo
Photo Courtesy of Files Laboratory, Wake Forest University
The in-vivo assay is perhaps the least practiced technique of the three, despite being described in literature for over 25 years. This assay measures the aggregate torque produced by either the plantar or dorsi flexor muscles of the lower limb. The animal is anaesthetized while its foot is attached to a pedal like boot, termed a ‘footplate’, which is fastened to a dual-mode lever. When preparing the animal, the tibia is aligned perpendicular to the lever and the hips are slightly raised. This permits the torsion to occur in line with the axis of rotation.
The muscle is stimulated by using subcutaneous EMG electrodes. The most technically challenging part of this assay is the optimal placement of the electrodes and stimulation parameters. To get the best results, the least amount of current required to get a maximal force reading is needed for stimulation. This inhibits current from “bleeding” out of the area of interest and partially stimulating another muscle group which may antagonize the function of the muscle being tested. Whether plantor or dorsi flexion is elicited will depend on which nerve is stimulated. The fibular or peroneal nerve is used for dorsi flexion, and the sciatic nerve for plantar.
When using the 3-in-1 system in an in-vivo configuration, we are actually measuring ankle torsion of the animal’s hind limb, as opposed to direct force. Torque measures the tendency of a force to rotate an object about an axis.
In these experiments, the measurement of torque is normalized to one of the following parameters:
- relative muscle mass
- animal mass
- estimates of cross-sectional area
Strengths
- high-throughput assay
- physiologically relevant
- no surgical isolation or neurovascular alteration
- longitudinal testing possible
Challenges
- determination of a muscle’s contribution to overall function may be challenging
- depends on intact neuromuscular junction
- placement of electrodes requires practice to achieve consistency
Research and Experimental Applications
The versatility of the 1300A 3-in-1 Whole Animal Muscle System enables researchers to perform a vast array of functional assays in order to test different aspects of muscle. These include assays of:
- contractile function
- isometric contraction
- concentric contraction
- fatigue
- injury susceptibility
- eccentric
- isometric
When conducting these experiments, there are two basic assumptions that we must consider: optimal muscle length and supra-maximal stimulation.
Optimal muscle length, or Lo, is the optimal length a muscle must be in order to produce its largest measurement of force. Supra-maximal stimulation is the minimum amount of current needed in order to ensure recruitment of all muscle fibres with a single action potential.
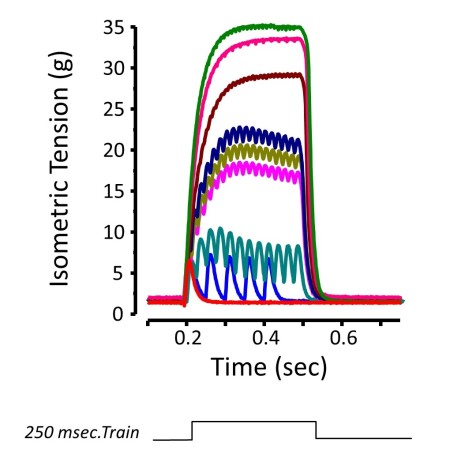
Isometric Contraction
Figure Courtesy of Ward & Khairallah
The most common functional assessment is the isometric contraction. This type of contraction occurs when force is produced with no change in muscle length. A real world conceptual example would be pushing against a wall. The classic way of assessing this type of contraction is by creating force vs. time curve while varying the frequency of stimulation. This is done by stimulating the muscle with trains of action potentials at increasing frequencies until there is no further increase in force measurement. The peak force responses for each stimulation frequency is determined and graphed.
Fatigue
Fatigue protocols are designed to evaluate the decrease in force of a muscle over time due to continuous stimulation. A practical example was cited in the webinar from a recent publication by the Lovering lab at the University of Maryland. The fatigue protocol was performed in-vivo. During the experiment the femoral nerve was stimulated every second for 2 mins with a 330-ms tetanic pulse. Additionally electrodes were used to directly stimulate the muscle itself and a tetanic contraction was superimposed every 15 seconds throughout the protocol, . This allows the researcher to dissect out the differences in phenotypic loss of force that result from both muscle and nerve stimulation.
Injury Susceptibility
Injury susceptibility is an important consideration for researchers in fields such as muscular disease. The study cited in the webinar by Khairallah et al., used the in-vivo technique. A tetanic stimulation was administered to the TA muscle long enough for the muscle’s force output to plateau. The muscle was then stretched while still being stimulated, creating an eccentric contraction, which induced muscle damage. In the study, a wild type mouse was compared to a mouse model for muscular dystrophy, the mdx transgenic mouse. The eccentric contractions were repeated 20 times and were applied to both mouse models. Differences in loss of force over the battery of contractions were observed. Because the protocol was applied in-vivo, both mouse models could be later tested to assess their recovery, with much less recovery shown by the mdx mouse due to its lack of a functional dystrophin protein.
We encourage everyone to view the webinar by clicking,
Three Techniques, One System: How to Effectively Characterize Complete Muscle Function
Stay tuned for more webinars from Aurora Scientific in the future!