The Canadian Neuroscience Meeting (CAN) is hosted in a different Canadian city each year, welcoming in researchers from across the country and beyond. With the 2025 meeting taking place close to our headquarters, Aurora Scientific is ecstatic to participate as an exhibitor. Our parent company, Lafayette Instrument, will also be attendance, along with its remaining life science brands – Campden Instruments, Actimetrics, and ALZET Osmotic Pumps. Together, we’re looking forward to serving researchers at home and across the globe. In a scent-imental tribute to our inaugural CAN attendance, the following publication review sniffs out recent olfaction studies, spanning the delineation of olfactory mechanisms to neurodevelopmental symptoms.
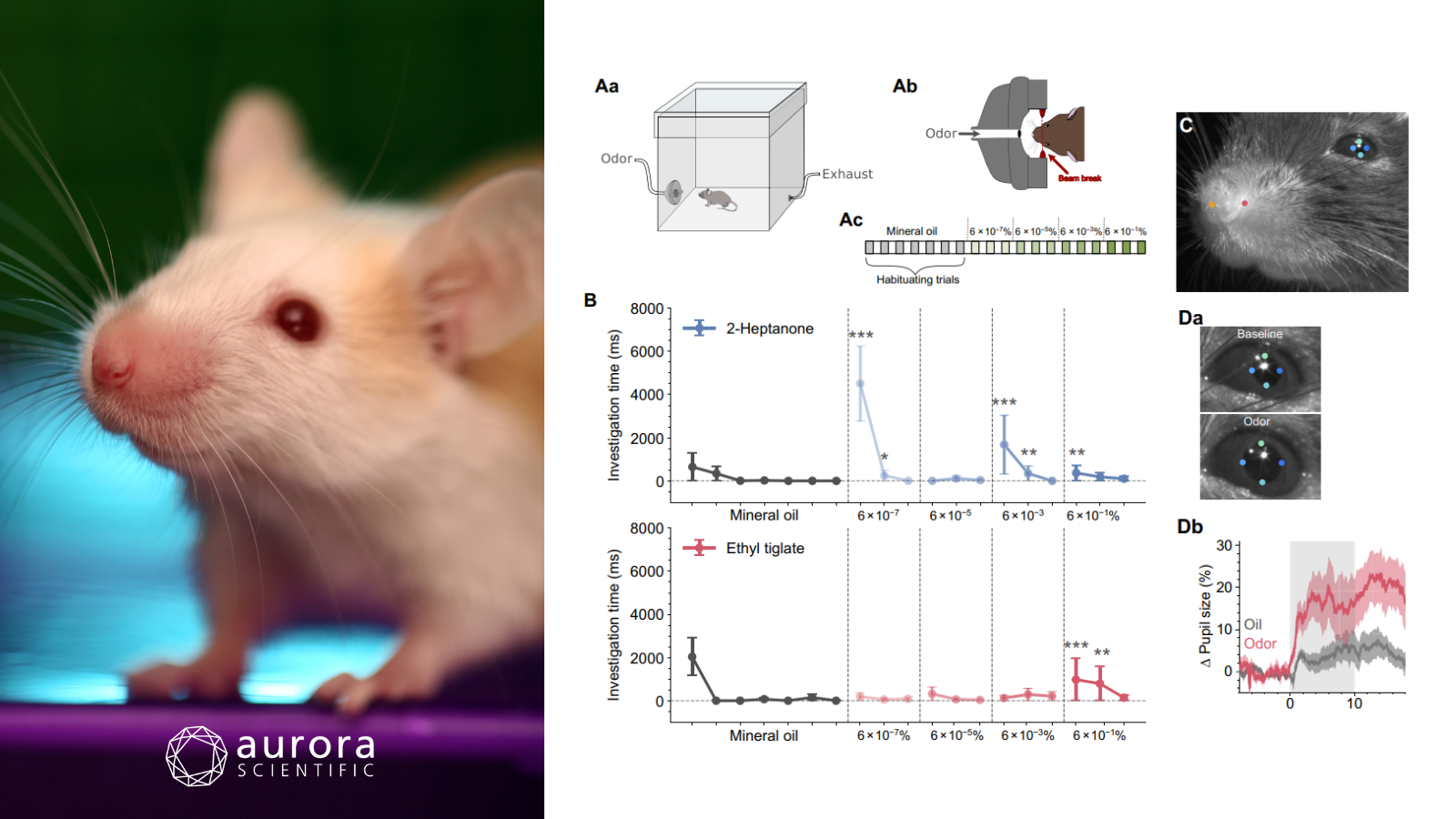
Featured image with a photo of a mouse (©DanBrandenburg from Getty Images Signature via Canva.com) and figures (adapted from ©Conway et al. (2024), licensed under CC BY 4.0) depicting concentration-dependent changes in olfactory perception. Aa) Experimental setup with a test chamber, odor delivery port, and exhaust, Ab) schematic of odor delivery port with a beam break sensor in the nose poke to log investigation times, (Ac) odor delivery protocol, B) odor investigation times during stimulus delivery for 2-heptanone (blue) and ethyl tiglate (red), C) facial feature tracking of head-fixed mice, Da) pupil diameter before and after odor stimulation, and Db) relative change in pupil diameter during ethyl tiglate presentation (red) and for three preceding stimulus blanks (grey), with stimulus period indicated by grey shaded area.
Perceptual constancy for an odor is acquired through changes in primary sensory neurons
Perceptual constancy refers to the ability to consistently recognize an object despite varying sensory input. While this phenomenon is well documented, the specific mechanisms by which perceptual constancy is achieved remains unclear. To uncover this, Conway et al. (2024) explored how mice perceive a naïve odour across varying concentrations, and how this perception shifts after association with the odour.
Diluted odorants were delivered using Aurora Scientific’s 220A: Olfactometer, maintaining a constant total flow rate of 1000 sccm (standard cubic centimeters per minute). Odors were presented in increasing concentrations and monitored using the 200C: miniPID Fast Response Olfaction Sensor, positioned near the odor source. To evaluate the mice’s novel odor detection, video recordings tracked key facial features in head-fixed mice on a treadmill. Additionally, in-vivo two-photon imaging was employed to examine how the brain represented the full range of odor concentrations.
Intriguingly, naïve mice were found to experience a concentration-dependent shift in odor perception due to transmission failure from olfactory receptor neurons at high concentrations. Responses from the primary glomerulus revealed that this was likely caused by the depolarizing block, which terminates spontaneous action potential firing. After associating an odor with food, this transmission failure was prevented, enabling stable perception across a wide range of concentrations. This perceptual stability was linked to plasticity in the primary sensory neurons, including changes in receptor sensitivity and glomerular structure. Taken together, these findings suggest that early olfactory experiences shape a sparse, stable odor identity code through adaptive mechanisms in the nasal epithelium, shedding light on how odor perceptual constancy is acquired.
Fast updating feedback from piriform cortex to the olfactory bulb relays multimodal identity and reward contingency signals during rule-reversal
Long-range feedforward and feedback interactions between brain regions are crucial for flexible behaviour. The olfactory bulb receives structured input from odorant receptors and sends signals via mitral and tufted cells to various cortical targets, which in turn, send feedback that shapes odor processing. While previous research has demonstrated that cortical feedback to the olfactory bulb is sparse, odor-specific, and strengthened by learning, it is unclear how this feedback adapts during sudden changes in reward associations to support behavioural flexibility. Therefore, Hernandez et al. (2025) examined whether piriform-to-olfactory bulb feedback axons encode changes in reward contingency, in addition to odor identity, during a rule-reversal task.
To assess this, head-fixed mice were trained to discriminate between two brief (350 ms) sensory cues: a pure tone target (Go) stimulus and a monomolecular odorant distractor (No-Go). Aurora Scientific’s 200C: miniPID Fast Response Olfaction Sensor was employed to select 1% ethyl valerate as an odorant cue due to its high SNR photoionization. Multiphoton imaging was conducted to capture neural activity in the olfactory bulb, while optogenetic suppression was performed in mice expressing the inhibitory opsin Jaws. These mice received light stimulation during select trials to inhibit cortical feedback.
Notably, feedback from the piriform cortex to the olfactory bulb conveyed both stimulus identity and reward context and was found to play a critical role in behavioural flexibility. Specifically, the cortical feedback axons responded to odors and auditory cues, and rapidly adapted their activity following changes in stimulus-reward contingencies. These neural changes preceded and aligned with behavioural adjustments. In fact, optogenetic suppression of the feedback pathway significantly impaired task performance, supporting its role in adaptive decision-making. As such, these findings reveal that cortical feedback dynamically reshapes olfactory processing to support adaptive decision-making in response to changing environmental demands.
Autistic behavior is a common outcome of biallelic disruption of PDZD8 in humans and mice
Intellectual disability with autism and dysmorphic facies (IDDADF) is a rare, neurodevelopmental disorder caused by mutations in the PDZD8 gene. These mutations perturb the PDZD8 protein involved in neuronal lipid transfer and calcium regulation. Given the limited number of clinical patients, mouse models for PDZD8-related ID, such as the Pdzd8tm1b mouse line, are critical for delineating disease pathophysiology. To this end, Pantiru et al. (2025) investigated the behavioural and neurological consequences of Pdzd8-deficiency in the Pdzd8tm1b mouse line, as well as a newly identified family with biallelic mutation of PDZD8 (known as family C).
A multitude of assessments, such as homozygosity mapping, whole-exome sequencing, and co-segregation analysis were performed to identify the PDZD8 variant responsible for IDDADF in consanguineous family C. In the Pdzd8tm1b mouse line, Aurora Scientific’s 220A: Olfactometer was used to deliver socially-relevant odors for olfactory detection, habituation, and social discrimination assessment. Moreover, behavioural, structural magnetic resonance imaging, and microscopy analyses were conducted, and metabolic activity was profiled using sealed metabolic cages.
Clinical investigations into family C revealed two siblings with severe intellectual disability, autism, facial dysmorphism, and additional neurological symptoms, all linked to a homozygous nonsense mutation in exon 1 of PDZD8. Male Pdzd8tm1b mice showed increased activity and metabolic rate despite reduced body weight, reflecting a PDZD8-linked metabolic phenotype that mirrors the reduced body size observed in the affected human siblings. Female Pdzd8tm1b mice exhibited specific deficits in social recognition and olfactory-based social behaviour, including the inability to discriminate between socially relevant odors, alongside reduced cerebellar nuclei volume, increased olfactory cortex volume, diminished adult neurogenesis, and increased dendritic spine density. Additionally, Pdzd8tm1b mice displayed elevated markers of endoplasmic reticulum stress and mitochondrial fusion in the brain. These findings collectively implicate PDZD8 in neurodevelopmental, metabolic, and cellular pathways underlying autism and intellectual disability.
Conclusions
As CAN fast approaches to platform the latest advances in neuroscience, these studies by Conway et al. (2024), Hernandez et al. (2025), and Pantiru et al. (2025) underscore the coordinated regulation of olfactory processing. From the exploration of peripheral sensory plasticity, dynamic cortical feedback, and genetic perturbation, they collectively highlight the olfactory system’s scent-ral role in cognition and neurodevelopment.