Tissue engineering is an important field of regenerative medicine for tissue repair after damage caused by disease or physical trauma. Stem cells are important tools due to their capacity to differentiate into a large number of cells according to the stimuli provided. Embryonic stem cells, adult stem cells, and induced pluripotent stem cells can be used for this purpose. Tissue engineering holds the promise of sustainable improvement in the quality of human life, with a reduction in the societal and economic cost associated with healthcare and life expectancy, especially with regards to organ and tissue transplantation. This publication review will address three articles that use our force transducers to assess the mechanics of three different engineered tissues, skeletal muscle, cardiac muscle, and tendon.
Hero Image sourced from Adobe Stock.
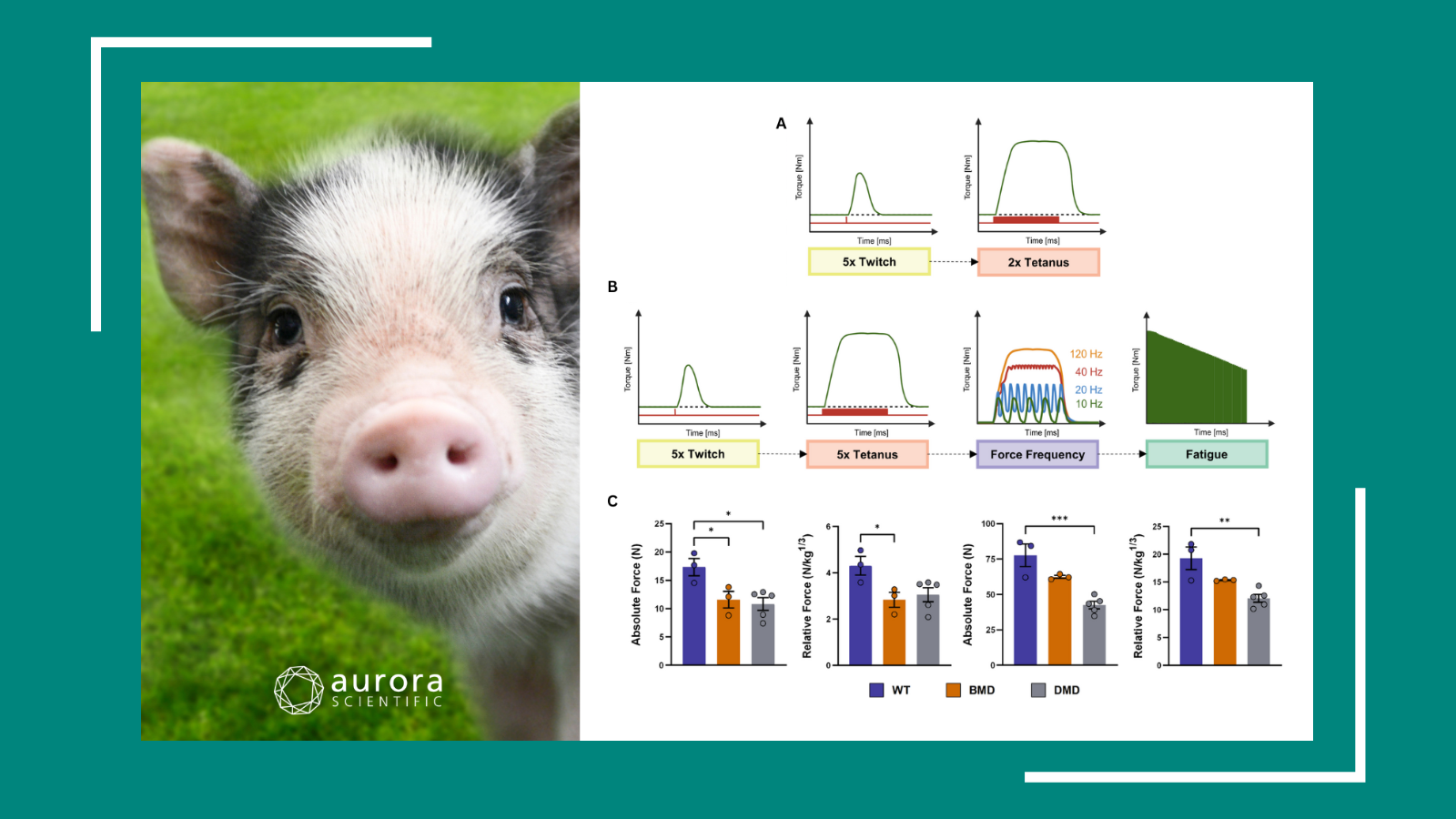
Physiological and pathophysiological concentrations of fatty acids induce lipid droplet accumulation and impair functional performance of tissue engineered skeletal muscle
M.C. Turner et al. (2020) designed these experiments to determine the effects of physiological and pathophysiological concentrations of exogenous fatty acids on the physiological performance of tissue engineered skeletal muscle (TESkM). TESkM constructs were generated using C2C12 myoblasts and attached to our 403A force transducer. Wire electrodes were positioned on either side of the construct to allow for electric field stimulation. Maximal twitch force and tetanic force were measured and the constructs were then processed for immunocytochemistry. Increasing concentrations of oleic, palmitic, linoleic, and α‐linoleic acids resulted in a significant reduction in absolute maximal force generation (tetanic force) compared to control. Single contraction (twitch) force production showed a similar pattern to tetanic force responses and was significantly lower when exposed to higher concentrations of these fatty acids. These findings show that engineered skeletal muscle is responsive to different concentrations of exogenous fatty acids due to changes in composition and lipid accumulation, and these changes are accompanied by the absolute reduction in functional capacity.
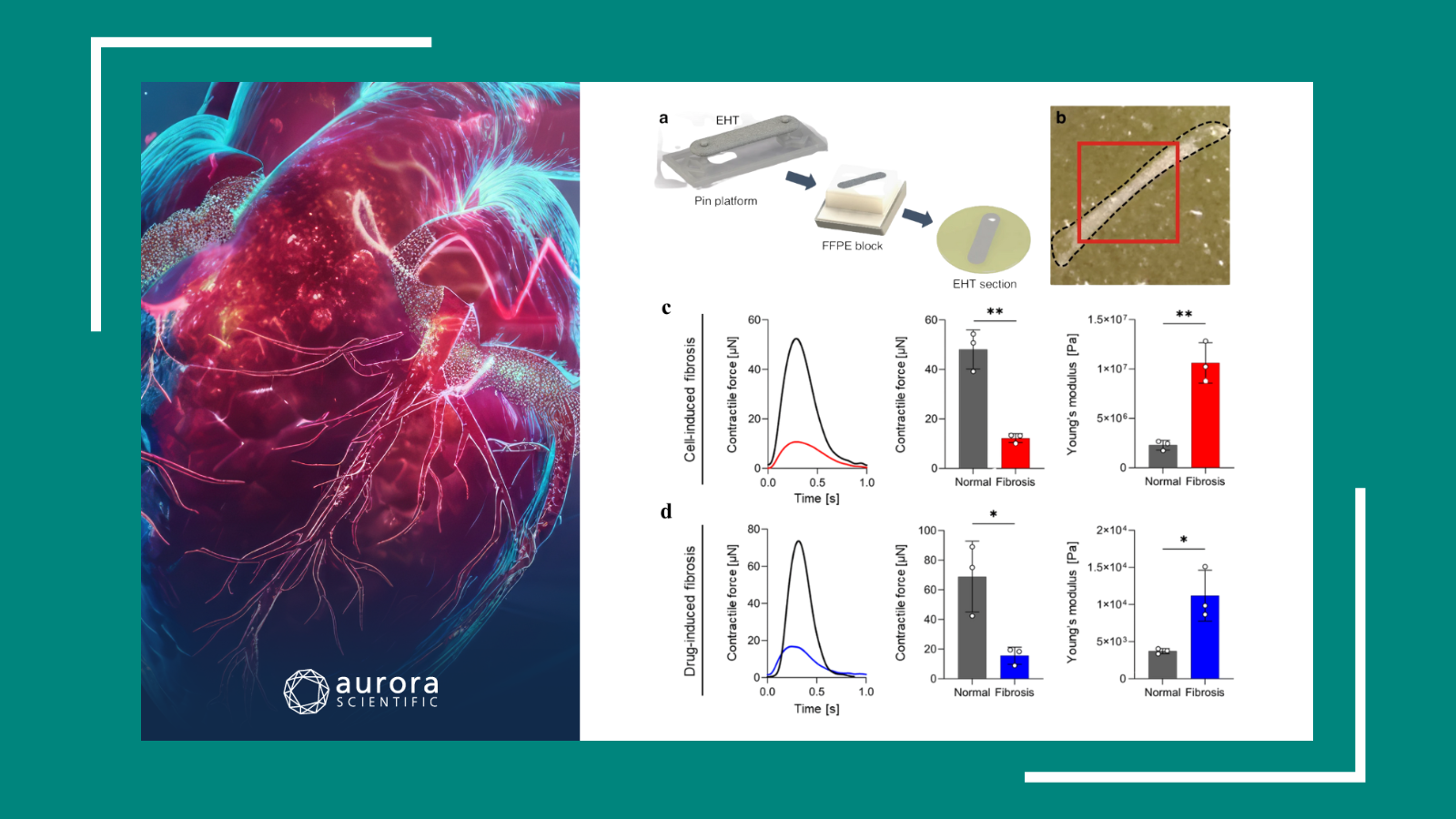
Human Cardiac Fibroblast Number and Activation State Modulate Electromechanical Function of hiPSC-Cardiomyocytes in Engineered Myocardium
This study by C.E. Rupert et al. (2020) evaluates the functional effects of culturing adult human cardiac fibroblasts (hCFs) in 3D engineered tissues on excitation and contraction with the goal of creating healthy myocardia in vitro. The cardiac tissue constructs were engineered with different amounts of hCFs to determine functional role of cellular interactions between hCFs and cardiomyocytes in the mechanical performance of engineered cardiac tissue. Mechanical measurements were performed on strips of engineered cardiac tissue, and their passive and active mechanical properties were measured with our 1500A isolated muscle system. The contractile force produced during twitch contractions increased significantly with the inclusion of 5% hCFs. However, tissues containing greater amounts of hCFs did not differ significantly from control. These results demonstrate that adult human cardiac fibroblasts (hCFs) can be used to optimize engineered cardiac tissue electromechanical performance and that a low percentage of dormant hCFs are a valuable cell source to advance a healthy electromechanical response of engineered cardiac tissue for regenerative medicine applications.
Effects of genipin crosslinking on mechanical cell-matrix interaction in 3D engineered tendon constructs
In this study, A. Giannopoulos et al. (2020) quantify the effects of matrix rigidity on matrix interactions by inducing cross-links or by blocking cross-link formation in engineered tendon constructs. Genepin was used to induce cross-link formation and beta-aminopropionitrile (BAPN) was used to block cross-link formation. The cell-matrix mechanics of the tendon constructs were evaluated using our 402A force transducers for a series of unloading/reloading cycles. The mechanical testing of tendon constructs showed increased peak stress at the highest genipin concentration but decreased peak stress with BAPN treatment when compared to controls. Tendon construct stiffness also increased with high genipin concentrations and decreased in BAPN-treated constructs, relative to the controls. These results suggest that human tendon fibroblasts regulate force exertion in a way that is inversely proportional to increased cross-link capacity but does so completely independently of matrix stiffness. Overall, these conclusions support the hypothesis that there is a relationship between cell force generation and cross-linking, and thus a role for this interaction in the mechanical homeostasis of the tissue.